- Record: found
- Abstract: found
- Article: found
Development of nanobodies targeting hepatocellular carcinoma and application of nanobody-based CAR-T technology
Read this article at
Abstract
Background
Chimeric antigen receptor T (CAR-T) cell therapy, as an emerging anti-tumor treatment, has garnered extensive attention in the study of targeted therapy of multiple tumor-associated antigens in hepatocellular carcinoma (HCC). However, the suppressive microenvironment and individual heterogeneity results in downregulation of these antigens in certain patients’ cancer cells. Therefore, optimizing CAR-T cell therapy for HCC is imperative.
Methods
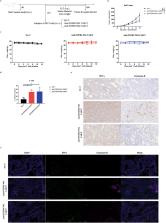
In this study, we administered FGFR4-ferritin (FGFR4-HPF) nanoparticles to the alpaca and constructed a phage library of nanobodies (Nbs) derived from alpaca, following which we screened for Nbs targeting FGFR4. Then, we conducted the functional validation of Nbs. Furthermore, we developed Nb-derived CAR-T cells and evaluated their anti-tumor ability against HCC through in vitro and in vivo validation.
Results
Our findings demonstrated that we successfully obtained high specificity and high affinity Nbs targeting FGFR4 after screening. And the specificity of Nbs targeting FGFR4 was markedly superior to their binding to other members of the FGFR family proteins. Furthermore, the Nb-derived CAR-T cells, targeting FGFR4, exhibited significantly enhanced anti-tumor efficacy in both experiments when in vitro and in vivo.
Conclusions
In summary, the results of this study suggest that the CAR-T cells derived from high specificity and high affinity Nbs, targeting FGFR4, exhibited significantly enhanced anti-tumor efficacy in vitro and in vivo. This is an exploration of FGFR4 in the field of Nb-derived CAR-T cell therapy for HCC, holding promise for enhancing safety and effectiveness in the clinical treatment of HCC in the future.
Related collections
Most cited references46
- Record: found
- Abstract: found
- Article: not found
Hepatocellular carcinoma: epidemiology and molecular carcinogenesis.
- Record: found
- Abstract: found
- Article: not found
4-1BB Costimulation Ameliorates T Cell Exhaustion Induced by Tonic Signaling of Chimeric Antigen Receptors
- Record: found
- Abstract: not found
- Article: not found