- Record: found
- Abstract: found
- Article: found
Localization of NG2 immunoreactive neuroglia cells in the rat locus coeruleus and their plasticity in response to stress
Read this article at
Abstract
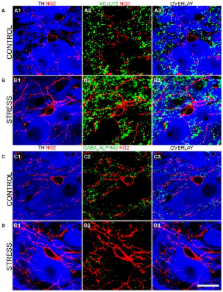
The locus coeruleus (LC) nucleus modulates adaptive behavioral responses to stress and dysregulation of LC neuronal activity is implicated in stress-induced mental illnesses. The LC is composed primarily of noradrenergic neurons together with various glial populations. A neuroglia cell-type largely unexplored within the LC is the NG2 cell. NG2 cells serve primarily as oligodendrocyte precursor cells throughout the brain. However, some NG2 cells are in synaptic contact with neurons suggesting a role in information processing. The aim of this study was to neurochemically and anatomically characterize NG2 cells within the rat LC. Furthermore, since NG2 cells have been shown to proliferate in response to traumatic brain injury, we investigated whether such NG2 cells plasticity also occurs in response to emotive insults such as stress. Immunohistochemistry and confocal microscopy revealed that NG2 cells were enriched within the pontine region occupied by the LC. Close inspection revealed that a sub-population of NG2 cells were located within unique indentations of LC noradrenergic somata and were immunoreactive for the neuronal marker NeuN whilst NG2 cell processes formed close appositions with clusters immunoreactive for the inhibitory synaptic marker proteins gephyrin and the GABA-A receptor alpha3-subunit, on noradrenergic dendrites. In addition, LC NG2 cell processes were decorated with vesicular glutamate transporter 2 immunoreactive puncta. Finally, 10 days of repeated restraint stress significantly increased the density of NG2 cells within the LC. The study demonstrates that NG2 IR cells are integral components of the LC cellular network and they exhibit plasticity as a result of emotive challenges.
Related collections
Most cited references60
- Record: found
- Abstract: found
- Article: not found
NG2-expressing glial progenitor cells: an abundant and widespread population of cycling cells in the adult rat CNS.
- Record: found
- Abstract: found
- Article: not found
Glutamatergic synapses on oligodendrocyte precursor cells in the hippocampus.
- Record: found
- Abstract: found
- Article: not found