- Record: found
- Abstract: found
- Article: found
Dynamic Modulation of Mouse Locus Coeruleus Neurons by Vasopressin 1a and 1b Receptors
Read this article at
Abstract
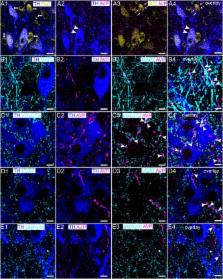
The locus coeruleus (LC) is a brainstem nucleus distinguished by its supply of noradrenaline throughout the central nervous system. Apart from modulating a range of brain functions, such as arousal, cognition and the stress response, LC neuronal excitability also corresponds to the activity of various peripheral systems, such as pelvic viscera and the cardiovascular system. Neurochemically diverse inputs set the tone for LC neuronal activity, which in turn modulates these adaptive physiological and behavioral responses essential for survival. One such LC afferent system which is poorly understood contains the neurohormone arginine-vasopressin (AVP). Here we provide the first demonstration of the molecular and functional characteristics of the LC-AVP system, by characterizing its receptor-specific modulation of identified LC neurons and plasticity in response to stress. High resolution confocal microscopy revealed that immunoreactivity for the AVP receptor 1b (V1b) was located on plasma membranes of noradrenergic and non-noradrenergic LC neurons. In contrast, immunoreactivity for the V1a receptor was exclusively located on LC noradrenergic neurons. No specific signal, either at the mRNA or protein level, was detected for the V2 receptor in the LC. Clusters immunoreactive for V1a-b were located in proximity to profiles immunoreactive for GABAergic and glutamatergic synaptic marker proteins. AVP immunopositive varicosities were also located adjacent to labeling for such synaptic markers. Whole-cell patch clamp electrophysiology revealed that the pharmacological activation of V1b receptors significantly increased the spontaneous activity of 45% (9/20) of recorded noradrenergic neurons, with the remaining 55% (11/20) of cells exhibiting a significant decrease in their basal firing patterns. Blockade of V1a and V1b receptors on their own significantly altered LC neuronal excitability in a similar heterogeneous manner, demonstrating that endogenous AVP sets the basal LC neuronal firing rates. Finally, exposing animals to acute stress increased V1b, but not V1a receptor expression, whilst decreasing AVP immunoreactivity. This study reveals the AVP-V1a-b system as a considerable component of the LC molecular architecture and regulator of LC activity. Since AVP primarily functions as a regulator of homeostasis, the data suggest a novel pathway by modulating the functioning of a brain region that is integral to mediating adaptive responses.
Related collections
Most cited references64
- Record: found
- Abstract: not found
- Article: not found
Characterization of a 41-residue ovine hypothalamic peptide that stimulates secretion of corticotropin and beta-endorphin
- Record: found
- Abstract: found
- Article: not found
CRH Engagement of the Locus Coeruleus Noradrenergic System Mediates Stress-Induced Anxiety.
- Record: found
- Abstract: found
- Article: not found