- Record: found
- Abstract: found
- Article: found
NG2 antigen is a therapeutic target for MLL-rearranged B-cell acute lymphoblastic leukemia
Read this article at
Abstract
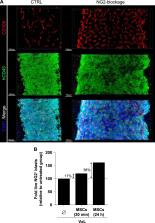
B cell acute lymphoblastic leukemia (B-ALL) is the most common childhood cancer, with cure rates of ∼80%. MLL-rearranged (MLLr) B-ALL (MLLr-B-ALL) has, however, an unfavorable prognosis with common therapy refractoriness and early relapse, and therefore new therapeutic targets are needed for relapsed/refractory MLLr-B-ALL. MLLr leukemias are characterized by the specific expression of chondroitin sulfate proteoglycan-4, also known as neuron-glial antigen-2 (NG2). NG2 was recently shown involved in leukemia invasiveness and central nervous system infiltration in MLLr-B-ALL, and correlated with lower event-free survival (EFS). We here hypothesized that blocking NG2 may synergize with established induction therapy for B-ALL based on vincristine, glucocorticoids, and l-asparaginase (VxL). Using robust patient-derived xenograft (PDX) models, we found that NG2 is crucial for MLLr-B-ALL engraftment upon intravenous (i.v.) transplantation. In vivo blockade of NG2 using either chondroitinase-ABC or an anti-NG2-specific monoclonal antibody (MoAb) resulted in a significant mobilization of MLLr-B-ALL blasts from bone marrow (BM) to peripheral blood (PB) as demonstrated by cytometric and 3D confocal imaging analysis. When combined with either NG2 antagonist, VxL treatment achieved higher rates of complete remission, and consequently higher EFS and delayed time to relapse. Mechanistically, anti-NG2 MoAb induces neither antibody-dependent cell-mediated not complement-dependent cytotoxicity. NG2 blockade rather overrides BM stroma-mediated chemoprotection through PB mobilization of MLLr-B-ALL blasts, thus becoming more accessible to chemotherapy. We provide a proof of concept for NG2 as a therapeutic target for MLLr-B-ALL.
Related collections
Most cited references53
- Record: found
- Abstract: found
- Article: found
Acute lymphoblastic leukemia: a comprehensive review and 2017 update
- Record: found
- Abstract: found
- Article: not found
Normal and leukemic stem cell niches: insights and therapeutic opportunities.
- Record: found
- Abstract: found
- Article: not found