- Record: found
- Abstract: found
- Article: found
M1 macrophage-derived exosomes inhibit cardiomyocyte proliferation through delivering miR-155
Read this article at
Abstract
Background
M1 macrophages are closely associated with cardiac injury after myocardial infarction (MI). Increasing evidence shows that exosomes play a key role in pathophysiological regulation after MI, but the role of M1 macrophage-derived exosomes (M1-Exos) in myocardial regeneration remains unclear. In this study, we explored the impact of M1 macrophage-derived exosomes on cardiomyocytes regeneration in vitro and in vivo.
Methods
M0 macrophages were induced to differentiate into M1 macrophages with GM-CSF (50 ng/mL) and IFN-γ (20 ng/mL). Then M1-Exos were isolated and co-incubated with cardiomyocytes. Cardiomyocyte proliferation was detected by pH3 or ki67 staining. Quantitative real-time PCR (qPCR) was used to test the level of miR-155 in macrophages, macrophage-derived exosomes and exosome-treated cardiomyocytes. MI model was constructed and LV-miR-155 was injected around the infarct area, the proliferation of cardiomyocytes was counted by pH3 or ki67 staining. The downstream gene and pathway of miR-155 were predicted and verified by dual-luciferase reporter gene assay, qPCR and immunoblotting analysis. IL-6 (50 ng/mL) was added to cardiomyocytes transfected with miR-155 mimics, and the proliferation of cardiomyocytes was calculated by immunofluorescence. The protein expressions of IL-6R, p-JAK2 and p-STAT3 were detected by Western blot.
Results
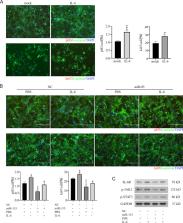
The results showed that M1-Exos suppressed cardiomyocytes proliferation. Meanwhile, miR-155 was highly expressed in M1-Exos and transferred to cardiomyocytes. miR-155 inhibited the proliferation of cardiomyocytes and antagonized the pro-proliferation effect of interleukin 6 (IL-6). Furthermore, miR-155 targeted gene IL-6 receptor (IL-6R) and inhibited the Janus kinase 2(JAK)/Signal transducer and activator of transcription (STAT3) signaling pathway.
Related collections
Most cited references24
- Record: found
- Abstract: not found
- Article: not found
Fourth Universal Definition of Myocardial Infarction (2018)
- Record: found
- Abstract: found
- Article: not found
Evidence for cardiomyocyte renewal in humans.
- Record: found
- Abstract: found
- Article: not found