- Record: found
- Abstract: found
- Article: found
Phage-DMS: A Comprehensive Method for Fine Mapping of Antibody Epitopes
Read this article at
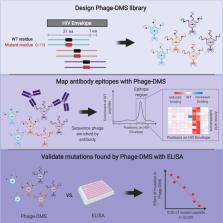
Summary
Understanding the antibody response is critical to developing vaccine and antibody-based therapies and has inspired the recent development of new methods to isolate antibodies. Methods to define the antibody-antigen interactions that determine specificity or allow escape have not kept pace. We developed Phage-DMS, a method that combines two powerful approaches—immunoprecipitation of phage peptide libraries and deep mutational scanning (DMS)—to enable high-throughput fine mapping of antibody epitopes. As an example, we designed sequences encoding all possible amino acid variants of HIV Envelope to create phage libraries. Using Phage-DMS, we identified sites of escape predicted using other approaches for four well-characterized HIV monoclonal antibodies with known linear epitopes. In some cases, the results of Phage-DMS refined the epitope beyond what was determined in previous studies. This method has the potential to rapidly and comprehensively screen many antibodies in a single experiment to define sites essential for binding interactions.
Graphical Abstract
Highlights
-
•
A high-throughput, comprehensive method to identify antibody epitopes is needed
-
•
Phage-DMS combines phage display technology and deep mutational scanning
-
•
Phage-DMS identified single mutations that lead to escape from HIV Env antibody binding
-
•
Effect of mutations in Phage-DMS correlate with results using a parallel approach
Abstract
Virology; Genomic Library
Related collections
Most cited references35
- Record: found
- Abstract: found
- Article: not found
Deep mutational scanning: a new style of protein science.
- Record: found
- Abstract: found
- Article: not found
High Resolution Mapping of Protein Sequence–Function Relationships
- Record: found
- Abstract: found
- Article: not found
