- Record: found
- Abstract: found
- Article: found
Social Network Strategies to Distribute HIV Self-testing Kits: A Global Systematic Review and Network Meta-analysis
Read this article at
Abstract
Introduction:
Social network strategies, in which social networks are utilized to influence individuals or communities, are increasingly being used to deliver human immunodeficiency virus (HIV) interventions to key populations. We summarized and critically assessed existing research on the effectiveness of social network strategies in promoting HIV self-testing (HIVST).
Methods:
Using search terms related to social network interventions and HIVST, we searched five databases for trials published between January 1 st, 2010, and June 30 th, 2023. Outcomes included uptake of HIV testing, HIV seroconversion, and linkage to antiretroviral therapy (ART) or HIV Care. We used network meta-analysis to assess the uptake of HIV testing through social network strategies compared with control methods. A pairwise meta-analysis of studies with a comparison arm that reported outcomes was performed to assess relative risks (RR) and their corresponding 95% confidence intervals (CI).
Results and discussion:
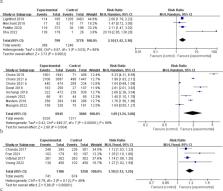
Among the 3,745 manuscripts identified, 33 studies fulfilled the inclusion criteria, including one quasi-experimental study, 17 RCTs and 15 observational studies. Networks HIVST testing was organized by peers (distributed to known peers, 15 studies), partners (distributed to their sexual partners, 10 studies), and peer educators (distributed to unknown peers, 8 studies). The results showed that all of the three social network distribution strategies enhanced the uptake of HIV testing compared to standard facility-based testing. Among social networks, peer distribution had the highest uptake of HIV testing (79% probability, SUCRA 0.92), followed by partner distribution (72% probability, SUCRA 0.71), and peer educator distribution (66% probability, SUCRA 0.29). Pairwise meta-analysis showed that peer distribution (RR 2.29, 95% CI 1.54–3.39, 5 studies) and partner distribution (RR 1.45, 95% CI 1.05–2.02, 7 studies) also increased the probability of detecting HIV reactivity during testing within the key population when compared to the control. Linkage to ART or HIV Care remained comparable to facility-based testing across the three HIVST distribution strategies.
Conclusions:
Network-based HIVST distribution is considered effective in augmenting HIV testing rates and reaching marginalized populations compared to facility-based testing. These strategies can be integrated with the existing HIV care services, to fill the testing gap among key populations globally.
PROSPERO Number: CRD42022361782
Related collections
Most cited references64

- Record: found
- Abstract: found
- Article: found
The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials
- Record: found
- Abstract: found
- Article: not found
The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations.
- Record: found
- Abstract: not found
- Article: not found