- Record: found
- Abstract: found
- Article: not found
Metal-Dependent DNA Recognition and Cell Internalization of Designed, Basic Peptides

Read this article at
Abstract

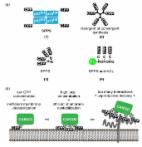
A fragment of the DNA basic region (br) of the GCN4 bZIP transcription factor has been modified to include two His residues at designed i and i+4 positions of its N-terminus. The resulting monomeric peptide ( brHis 2 ) does not bind to its consensus target DNA site (5′-GTCAT-3′). However, addition of Pd(en)Cl 2 (en, ethylenediamine) promotes a high-affinity interaction with exquisite selectivity for this sequence. The peptide–DNA complex is disassembled by addition of a slight excess of a palladium chelator, and the interaction can be reversibly switched multiple times by playing with controlled amounts of either the metal complex or the chelator. Importantly, while the peptide brHis 2 fails to translocate across cell membranes on its own, addition of the palladium reagent induces an efficient cell internalization of this peptide. In short, we report (1) a designed, short peptide that displays highly selective, major groove DNA binding, (2) a reversible, metal-dependent DNA interaction, and (3) a metal-promoted cell internalization of this basic peptide.
Related collections
Most cited references30
- Record: found
- Abstract: found
- Article: not found
Spectroscopic methods for analysis of protein secondary structure.
- Record: found
- Abstract: found
- Article: not found
Cell internalization of the third helix of the Antennapedia homeodomain is receptor-independent.
- Record: found
- Abstract: found
- Article: found
