- Record: found
- Abstract: found
- Article: found
Acid Stress Response Mechanisms of Group B Streptococci
Read this article at
Abstract
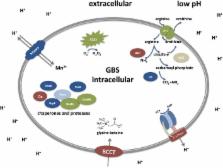
Group B streptococcus (GBS) is a leading cause of neonatal mortality and morbidity in the United States and Europe. It is part of the vaginal microbiota in up to 30% of pregnant women and can be passed on to the newborn through perinatal transmission. GBS has the ability to survive in multiple different host niches. The pathophysiology of this bacterium reveals an outstanding ability to withstand varying pH fluctuations of the surrounding environments inside the human host. GBS host pathogen interations include colonization of the acidic vaginal mucosa, invasion of the neutral human blood or amniotic fluid, breaching of the blood brain barrier as well as survival within the acidic phagolysosomal compartment of macrophages. However, investigations on GBS responses to acid stress are limited. Technologies, such as whole genome sequencing, genome-wide transcription and proteome mapping facilitate large scale identification of genes and proteins. Mechanisms enabling GBS to cope with acid stress have mainly been studied through these techniques and are summarized in the current review
Related collections
Most cited references140
- Record: found
- Abstract: found
- Article: not found
Genome analysis of multiple pathogenic isolates of Streptococcus agalactiae: implications for the microbial "pan-genome".
- Record: found
- Abstract: found
- Article: not found
Fluorescence probe measurement of the intralysosomal pH in living cells and the perturbation of pH by various agents.
- Record: found
- Abstract: found
- Article: not found