- Record: found
- Abstract: found
- Article: found
Post-exposure prophylaxis with SA58 (anti-SARS-COV-2 monoclonal antibody) nasal spray for the prevention of symptomatic COVID-19 in healthy adult workers: a randomized, single-blind, placebo-controlled clinical study*
Read this article at
ABSTRACT
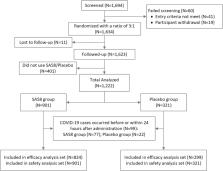
Monoclonal antibodies (mAbs) and the post-exposure prophylaxis (PEP) with mAbs represent a very important public health strategy against coronavirus disease 2019 (COVID-19). This study has assessed a new Anti-SARS-COV-2 mAb (SA58) Nasal Spray for PEP against COVID-19 in healthy adults aged 18 years and older within three days of exposure to a SARS-CoV-2 infected individual. Recruited participants were randomized in a ratio of 3:1 to receive SA58 or placebo. Primary endpoints were laboratory-confirmed symptomatic COVID-19 within the study period. A total of 1222 participants were randomized and dosed (SA58, n = 901; placebo, n = 321). Median of follow-up was 2.25 and 2.79 days for SA58 and placebo, respectively. Adverse events occurred in 221 of 901 (25%) and 72 of 321 (22%) participants with SA58 and placebo, respectively. All adverse events were mild in severity. Laboratory-confirmed symptomatic COVID-19 developed in 7 of 824 participants (0.22 per 100 person-days) in the SA58 group vs. 14 of 299 (1.17 per 100 person-days) in the placebo group, resulting in an estimated efficacy of 80.82% (95%CI 52.41%−92.27%). There were 32 SARS-CoV-2 reverse transcriptase polymerase chain reaction (RT–PCR) positives (1.04 per 100 person-days) in the SA58 group vs. 32 (2.80 per 100 person-days) in the placebo group, resulting in an estimated efficacy of 61.83% (95%CI 37.50%−76.69%). A total of 21 RT–PCR positive samples were sequenced and all were the Omicron variant BF.7. In conclusion, SA58 Nasal Spray showed favourable efficacy and safety in preventing symptomatic COVID-19 or SARS-CoV-2 infection in adults who had exposure to SARS-CoV-2 within 72 h.
Related collections
Most cited references14
- Record: found
- Abstract: found
- Article: not found
A Novel Coronavirus from Patients with Pneumonia in China, 2019
- Record: found
- Abstract: found
- Article: not found
The Incubation Period of Coronavirus Disease 2019 (COVID-19) From Publicly Reported Confirmed Cases: Estimation and Application

- Record: found
- Abstract: found
- Article: found