- Record: found
- Abstract: found
- Article: found
Boosting Protein Encapsulation through Lewis-Acid-Mediated Metal–Organic Framework Mineralization: Toward Effective Intracellular Delivery

Read this article at
Abstract
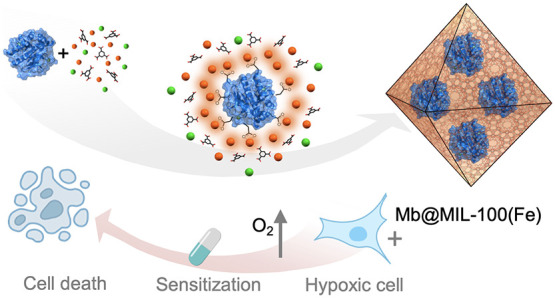
Encapsulation of biomolecules using metal–organic frameworks (MOFs) to form stable biocomposites has been demonstrated to be a valuable strategy for their preservation and controlled release, which has been however restricted to specific electrostatic surface conditions. We present a Lewis-acid-mediated general in situ strategy that promotes the spontaneous MOF growth on a broad variety of proteins, for the first time, regardless of their surface nature. We demonstrate that MOFs based on cations exhibiting considerable inherent acidity such as MIL-100(Fe) enable efficient biomolecule encapsulation, including elusive alkaline proteins previously inaccessible by the well-developed in situ azolate-based MOF encapsulation. Specifically, we prove the MIL-100(Fe) scaffold for the encapsulation of a group of proteins exhibiting very different isoelectric points (5 < pI < 11), allowing triggered release under biocompatible conditions and retaining their activity after exposure to denaturing environments. Finally, we demonstrate the potential of the myoglobin-carrying biocomposite to facilitate the delivery of O 2 into hypoxic human lung carcinoma A549 cells, overcoming hypoxia-associated chemoresistance.
Related collections
Most cited references55
- Record: found
- Abstract: not found
- Article: not found
Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity (Recommendations 1984)
- Record: found
- Abstract: not found
- Article: not found
Adsorption of Gases in Multimolecular Layers
- Record: found
- Abstract: found
- Article: not found