- Record: found
- Abstract: found
- Article: found
Metabolic rewiring in the promotion of cancer metastasis: mechanisms and therapeutic implications
Read this article at
Abstract
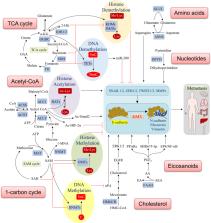
Tumor metastasis is the major cause of mortality from cancer. Metabolic rewiring and the metastatic cascade are highly intertwined, co-operating to promote multiple steps of cancer metastasis. Metabolites generated by cancer cells influence the metastatic cascade, encompassing epithelial-mesenchymal transition (EMT), survival of cancer cells in circulation, and metastatic colonization at distant sites. A variety of molecular mechanisms underlie the prometastatic effect of tumor-derived metabolites, such as epigenetic deregulation, induction of matrix metalloproteinases (MMPs), promotion of cancer stemness, and alleviation of oxidative stress. Conversely, metastatic signaling regulates expression and activity of rate-limiting metabolic enzymes to generate prometastatic metabolites thereby reinforcing the metastasis cascade. Understanding the complex interplay between metabolism and metastasis could unravel novel molecular targets, whose intervention could lead to improvements in the treatment of cancer. In this review, we summarized the recent discoveries involving metabolism and tumor metastasis, and emphasized the promising molecular targets, with an update on the development of small molecule or biologic inhibitors against these aberrant situations in cancer.
Related collections
Most cited references116
- Record: found
- Abstract: found
- Article: not found
Cabozantinib (XL184), a novel MET and VEGFR2 inhibitor, simultaneously suppresses metastasis, angiogenesis, and tumor growth.
- Record: found
- Abstract: found
- Article: not found