- Record: found
- Abstract: found
- Article: found
Lipids in cancer: a global view of the contribution of lipid pathways to metastatic formation and treatment resistance
Read this article at
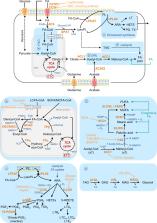
Abstract
Lipids are essential constituents for malignant tumors, as they are absolutely required for tumor growth and dissemination. Provided by the tumor microenvironment (TME) or by cancer cells themselves through activation of de novo synthesis pathways, they orchestrate a large variety of pro-tumorigenic functions. Importantly, TME cells, especially immune cells, cancer-associated fibroblasts (CAFs) and cancer-associated adipocytes (CAAs), are also prone to changes in their lipid content, which hinder or promote tumor aggressiveness. In this review, we address the significant findings for lipid contribution in tumor progression towards a metastatic disease and in the poor response to therapeutic treatments. We also highlight the benefits of targeting lipid pathways in preclinical models to slow down metastasis development and overcome chemo-and immunotherapy resistance.
Related collections
Most cited references153

- Record: found
- Abstract: found
- Article: found
A framework for advancing our understanding of cancer-associated fibroblasts

- Record: found
- Abstract: found
- Article: found