- Record: found
- Abstract: found
- Article: found
Cell migration through three-dimensional confining pores: speed accelerations by deformation and recoil of the nucleus
Read this article at
Abstract
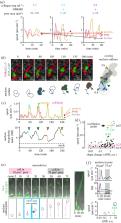
Directional cell migration in dense three-dimensional (3D) environments critically depends upon shape adaptation and is impeded depending on the size and rigidity of the nucleus. Accordingly, the nucleus is primarily understood as a physical obstacle; however, its pro-migratory functions by stepwise deformation and reshaping remain unclear. Using atomic force spectroscopy, time-lapse fluorescence microscopy and shape change analysis tools, we determined the nuclear size, deformability, morphology and shape change of HT1080 fibrosarcoma cells expressing the Fucci cell cycle indicator or being pre-treated with chromatin-decondensating agent TSA. We show oscillating peak accelerations during migration through 3D collagen matrices and microdevices that occur during shape reversion of deformed nuclei (recoil), and increase with confinement. During G1 cell-cycle phase, nucleus stiffness was increased and yielded further increased speed fluctuations together with sustained cell migration rates in confinement when compared to interphase populations or to periods of intrinsic nuclear softening in the S/G2 cell-cycle phase. Likewise, nuclear softening by pharmacological chromatin decondensation or after lamin A/C depletion reduced peak oscillations in confinement. In conclusion, deformation and recoil of the stiff nucleus contributes to saltatory locomotion in dense tissues.
This article is part of a discussion meeting issue ‘Forces in cancer: interdisciplinary approaches in tumour mechanobiology’.
Related collections
Most cited references35
- Record: found
- Abstract: found
- Article: not found
Nuclear mechanics during cell migration.
- Record: found
- Abstract: found
- Article: not found
Nuclear envelope composition determines the ability of neutrophil-type cells to passage through micron-scale constrictions.
- Record: found
- Abstract: found
- Article: not found