- Record: found
- Abstract: found
- Article: found
F-18 labelled PSMA-1007: biodistribution, radiation dosimetry and histopathological validation of tumor lesions in prostate cancer patients
Read this article at
Abstract
Purpose
The prostate-specific membrane antigen (PSMA) targeted positron-emitting-tomography (PET) tracer 68Ga-PSMA-11 shows great promise in the detection of prostate cancer. However, 68Ga has several shortcomings as a radiolabel including short half-life and non-ideal energies, and this has motivated consideration of 18F-labelled analogs. 18F-PSMA-1007 was selected among several 18F-PSMA-ligand candidate compounds because it demonstrated high labelling yields, outstanding tumor uptake and fast, non-urinary background clearance. Here, we describe the properties of 18F-PSMA-1007 in human volunteers and patients.
Methods
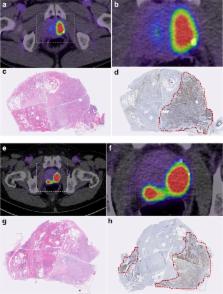
Radiation dosimetry of 18F-PSMA-1007 was determined in three healthy volunteers who underwent whole-body PET-scans and concomitant blood and urine sampling. Following this, ten patients with high-risk prostate cancer underwent 18F-PSMA-1007 PET/CT (1 h and 3 h p.i.) and normal organ biodistribution and tumor uptakes were examined. Eight patients underwent prostatectomy with extended pelvic lymphadenectomy. Uptake in intra-prostatic lesions and lymph node metastases were correlated with final histopathology, including PSMA immunostaining.
Results
With an effective dose of approximately 4.4–5.5 mSv per 200–250 MBq examination, 18F-PSMA-1007 behaves similar to other PSMA-PET agents as well as to other 18F-labelled PET-tracers. In comparison to other PSMA-targeting PET-tracers, 18F-PSMA-1007 has reduced urinary clearance enabling excellent assessment of the prostate. Similar to 18F-DCFPyL and with slightly slower clearance kinetics than PSMA-11, favorable tumor-to-background ratios are observed 2–3 h after injection. In eight patients, diagnostic findings were successfully validated by histopathology. 18F-PSMA-1007 PET/CT detected 18 of 19 lymph node metastases in the pelvis, including nodes as small as 1 mm in diameter.
Related collections
Most cited references11
- Record: found
- Abstract: found
- Article: not found
Initial Evaluation of [(18)F]DCFPyL for Prostate-Specific Membrane Antigen (PSMA)-Targeted PET Imaging of Prostate Cancer.
- Record: found
- Abstract: found
- Article: not found