- Record: found
- Abstract: found
- Article: found
Pharmacokinetic Study of Delavinone in Mice after Intravenous and Oral Administration by UPLC-MS/MS
Read this article at
Abstract
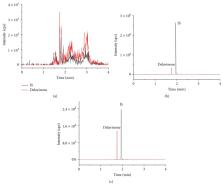
Thirty-one compounds, including delavinone, were isolated from the methanol extract of F. cirrhosa by modern chromatographic techniques. The pharmacological action of Fritillaria is widely used in clinical practice. However, the pharmacokinetic studies on delavinone have not been reported. Therefore, the chemical constituents of this species were investigated. Therefore, it is necessary to establish an analytical method to monitor the concentration of delavinone. An UPLC-MS/MS method was established to determine delavinone in the mouse blood, and the pharmacokinetics of delavinone after intravenous (1.0 mg/kg) and intragastric (2.5, 10.0 mg/kg) administration were studied. The lower limit of quantification was 1.0 ng/mL. The intraday and interday precision RSD were less than 13%, the accuracy ranged from 96.8% to 104.9%, the average recovery was better than 80.6%, and the matrix effect was between 88.8% and 103.4%. The UPLC-MS/MS method has been successfully applied to the pharmacokinetics of delavinone in mice. The noncompartment model was used to fit the main pharmacokinetic parameters. It was found that AUC in mice was higher than that in mice given orally, and the bioavailability of delavinone was 12.4%.
Related collections
Most cited references20
- Record: found
- Abstract: not found
- Article: not found
Investigation of association of chemical profiles with the tracheobronchial relaxant activity of Chinese medicinal herb Beimu derived from various Fritillaria species
- Record: found
- Abstract: not found
- Article: not found
Determination and pharmacokinetics of engeletin in rat plasma by ultra-high performance liquid chromatography with tandem mass spectrometry

- Record: found
- Abstract: found
- Article: found