- Record: found
- Abstract: found
- Article: found
Effects of Voriconazole on the Pharmacokinetics of Vonoprazan in Rats
Abstract
Purpose
The purpose of this study was to examine the effects of voriconazole on the pharmacokinetics of vonoprazan.
Methods
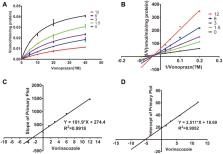
Fifteen Sprague-Dawley rats were randomly divided into three groups: five rats in each group, including control group, single-dose group (a single dose of 30 mg/kg of voriconazole), and multiple-dose group (multiple doses of 30 mg/(kg•day) per dose of voriconazole). Each group of rats was given an oral dose of 10 mg/kg vonoprazan 30 min after the administration of voriconazole or vehicle. After the oral administration of vonoprazan, 50 µL of blood was collected into 1.5-mL heparinized tubes via the caudal vein. The concentration of vonoprazan in plasma was quantified by ultra-performance liquid chromatography/tandem mass spectrometry. Both in vitro effects of voriconazole on vonoprazan and the mechanism of the observed inhibition were studied in rat liver microsomes.
Results
When orally administered, voriconazole increased the area under the plasma concentration–time curve (AUC), prolonged the elimination half-life (t 1/2), and decreased the clearance (CL) of vonoprazan; there was no significant difference between the single-dose and multiple-dose groups. Voriconazole inhibited the metabolism of vonoprazan at an IC50 of 2.93 μM and showed mixed inhibition. The results of the in vivo experiments were consistent with those of the in vitro experiments.
Conclusion
Our findings provide the evidence of drug–drug interactions between voriconazole and vonoprazan that could occur with pre-administration of voriconazole. Thus, clinicians should pay attention to the resulting changes in pharmacokinetic parameters and accordingly, adjust the dose of vonoprazan in clinical settings.
Most cited references43

- Record: found
- Abstract: found
- Article: found
Randomised clinical trial: vonoprazan, a novel potassium‐competitive acid blocker, vs. lansoprazole for the healing of erosive oesophagitis
- Record: found
- Abstract: found
- Article: not found