- Record: found
- Abstract: found
- Article: not found
Acinar Cell Apoptosis in Serpini2-Deficient Mice Models Pancreatic Insufficiency
Read this article at
Abstract
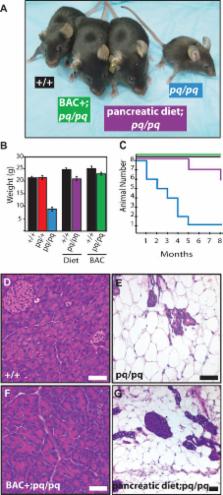
Pancreatic insufficiency (PI) when left untreated results in a state of malnutrition due to an inability to absorb nutrients. Frequently, PI is diagnosed as part of a larger clinical presentation in cystic fibrosis or Shwachman–Diamond syndrome. In this study, a mouse model for isolated exocrine PI was identified in a mouse line generated by a transgene insertion. The trait is inherited in an autosomal recessive pattern, and homozygous animals are growth retarded, have abnormal immunity, and have reduced life span. Mice with the disease locus, named pequeño (pq), exhibit progressive apoptosis of pancreatic acinar cells with severe exocrine acinar cell loss by 8 wk of age, while the islets and ductal tissue persist. The mutation in pq/pq mice results from a random transgene insertion. Molecular characterization of the transgene insertion site by fluorescent in situ hybridization and genomic deletion mapping identified an approximately 210-kb deletion on Chromosome 3, deleting two genes. One of these genes, Serpini2, encodes a protein that is a member of the serpin family of protease inhibitors. Reintroduction of only the Serpini2 gene by bacterial artificial chromosome transgenic complementation corrected the acinar cell defect as well as body weight and immune phenotypes, showing that deletion of Serpini2 causes the pequeño phenotype. Dietary supplementation of pancreatic enzymes also corrected body size, body weight, and immunodeficiency, and increased the life span of Serpini2-deficient mice, despite continued acinar cell loss. To our knowledge, this study describes the first characterized genetic animal model for isolated PI. Genetic complementation of the transgene insertion mutant demonstrates that Serpini2 deficiency directly results in the acinar cell apoptosis, malabsorption, and malnutrition observed in pq/pq mice. The rescue of growth retardation, immunodeficiency, and mortality by either Serpini2 bacterial artificial chromosome transgenic expression or by pancreatic enzyme supplementation demonstrates that these phenotypes are secondary to malnutrition in pq/pq mice.
Abstract
Synopsis
Pancreatic insufficiency is defined by the inability to digest and absorb nutrients due to the loss of pancreatic enzyme function or loss of the acinar cells that produce the enzymes. In this manuscript the authors have described a mouse model of pancreatic insufficiency characterized by the specific loss of pancreatic acinar cells. This specific acinar cell loss results in mice that are unable to digest and absorb nutrients from the diet, stunting the animal's growth and giving rise to immunological anomalies. The authors have identified a serendipitous transgene insertion/deletion encompassing the mouse Serpini2 gene locus as the source of the phenotypes observed. Reintroduction of the Serpini2 gene, a member of the serpin family of serine cysteine protease inhibitors, by bacterial artificial chromosome complementation corrects the pancreatic and immunological phenotypes of the disorder, confirming Serpini2 as the responsible gene. Reintroduction of pancreatic enzymes through diet supplementation is also capable of correcting the reduction in size and weight, reduction in viability, and immunological deficiencies, indicating that these phenotypes are secondary to malnutrition alone. This work provides a new mouse model for investigation of malnutrition/malabsorption due to pancreatic insufficiency and identifies a novel function for the serpin family member Serpini2.
Related collections
Most cited references33
- Record: found
- Abstract: found
- Article: not found
Resolution and characterization of pro-B and pre-pro-B cell stages in normal mouse bone marrow
- Record: found
- Abstract: found
- Article: not found
Leptin in the regulation of immunity, inflammation, and hematopoiesis.
- Record: found
- Abstract: found
- Article: not found
