- Record: found
- Abstract: found
- Article: not found
Coordination of Rho GTPase activities during cell protrusion
Read this article at
Abstract
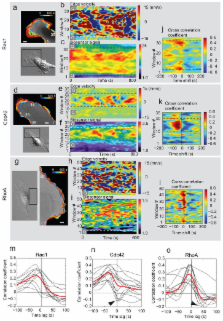
The GTPases Rac1, RhoA and Cdc42 act in concert to control cytoskeleton dynamics 1- 3. Recent biosensor studies have shown that all three GTPases are activated at the front of migrating cells 4- 7 and biochemical evidence suggests that they may regulate one another: Cdc42 can activate Rac1 8, and Rac1 and RhoA are mutually inhibitory 9- 12. However, their spatiotemporal coordination, at the seconds and single micron dimensions typical of individual protrusion events, remains unknown. Here, we examine GTPase coordination both through simultaneous visualization of two GTPase biosensors and using a “computational multiplexing” approach capable of defining the relationships between multiple protein activities visualized in separate experiments. We found that RhoA is activated at the cell edge synchronous with edge advancement, whereas Cdc42 and Rac1 are activated 2 μm behind the edge with a delay of 40 sec. This indicates that Rac1 and RhoA operate antagonistically through spatial separation and precise timing, and that RhoA plays a role in the initial events of protrusion, while Rac1 and Cdc42 activate pathways implicated in reinforcement and stabilization of newly expanded protrusions.
Related collections
Most cited references20
- Record: found
- Abstract: found
- Article: not found
Localized Rac activation dynamics visualized in living cells.
- Record: found
- Abstract: found
- Article: not found
Interplay between Rac and Rho in the control of substrate contact dynamics.
- Record: found
- Abstract: found
- Article: not found
