- Record: found
- Abstract: found
- Article: found
Modified regimen intrapleural alteplase with pulmozyme in pleural infection management: a tertiary teaching hospital experience
Read this article at
Abstract
Background
Current management of poorly draining complex effusions favours less invasive image-guided placement of smaller tubes and adjunctive intrapleural fibrinolysis therapy (IPFT). In MIST-2 trial, intrapleural 10 mg alteplase (t-PA) with 5 mg of pulmozyme (DNase) twice daily for 72 h were used. We aimed to assess the effectiveness and safety of a modified regimen 16 mg t-PA with 5 mg of DNase administered over 24 h in the management of complex pleural infection.
Methods
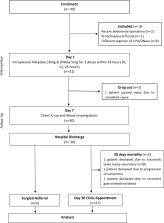
This was a single centre, prospective study involving patients with poorly drained pleural infection. Primary outcome was the change of pleural opacity on chest radiograph at day 7 compared to baseline. Secondary outcomes include volume of fluid drained, inflammatory markers improvement, surgical referral, length of hospitalisation, and adverse events.
Results
Thirty patients were recruited. Majority, 27 (90%) patients were successfully treated. Improvement of pleural opacity on chest radiograph was observed from 36.9% [Interquartile range (IQR 21.8–54.9%)] to 18.1% (IQR 8.8–32.7%) of hemithorax ( P < 0.05). T-PA/DNase increased fluid drainage from median of 45 mls (IQR 0–100) 24 h prior to intrapleural treatment to 1442 mls (IQR 905–2360) after 72 h; ( P < 0.05) and reduction of C-reactive protein ( P < 0.05). Pain requiring escalation of analgesia affected 20% patients and 9.9% experienced major adverse events. None required surgical intervention.
Conclusion
This study suggests that a modified regimen 16 mg t-PA with 5 mg DNase can be safe and effective for patients with poorly drained complex pleural infection.
Trial registration The study was registered retrospectively on 07/06/2021 with ClinicalTrials number NCT04915586 ( https://clinicaltrials.gov/ct2/show/NCT04915586).
Related collections
Most cited references25
- Record: found
- Abstract: not found
- Article: not found
Management of pleural infection in adults: British Thoracic Society Pleural Disease Guideline 2010.
- Record: found
- Abstract: found
- Article: not found
U.K. Controlled trial of intrapleural streptokinase for pleural infection.
- Record: found
- Abstract: found
- Article: not found