- Record: found
- Abstract: found
- Article: not found
Collective and Individual Migration following the Epithelial-Mesenchymal Transition
Read this article at
Abstract
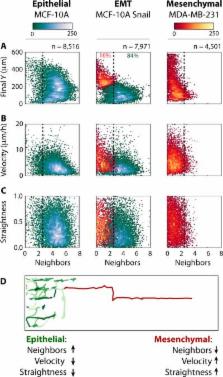
During cancer progression, malignant cells in the tumour invade surrounding tissues. This transformation of adherent cells to a motile phenotype has been associated with the epithelial mesenchymal transition (EMT). Here, we show that EMT-activated cells migrate through micropillar arrays as a collectively advancing front that scatters individual cells. Individual cells with few neighbours dispersed with fast, straight trajectories, whereas cells that encountered many neighbours migrated collectively with epithelial biomarkers. We modelled these emergent dynamics using a physical analogy to solidification phase transitions in binary mixtures, and validated it using drug perturbations, which revealed that individually migrating cells exhibit diminished chemosensitivity. Our measurements also indicate a degree of phenotypic plasticity as cells interconvert between individual and collective migration. The study of multicellular behaviours with single-cell resolution should enable further quantitative insights into heterogeneous tumour invasion.
Related collections
Most cited references33
- Record: found
- Abstract: found
- Article: not found
Epithelial-mesenchymal transitions in development and disease.
- Record: found
- Abstract: found
- Article: not found
Collective migration of an epithelial monolayer in response to a model wound.
- Record: found
- Abstract: found
- Article: not found
