- Record: found
- Abstract: found
- Article: found
Oral delivery of proteins and peptides: Challenges, status quo and future perspectives
Read this article at
Abstract
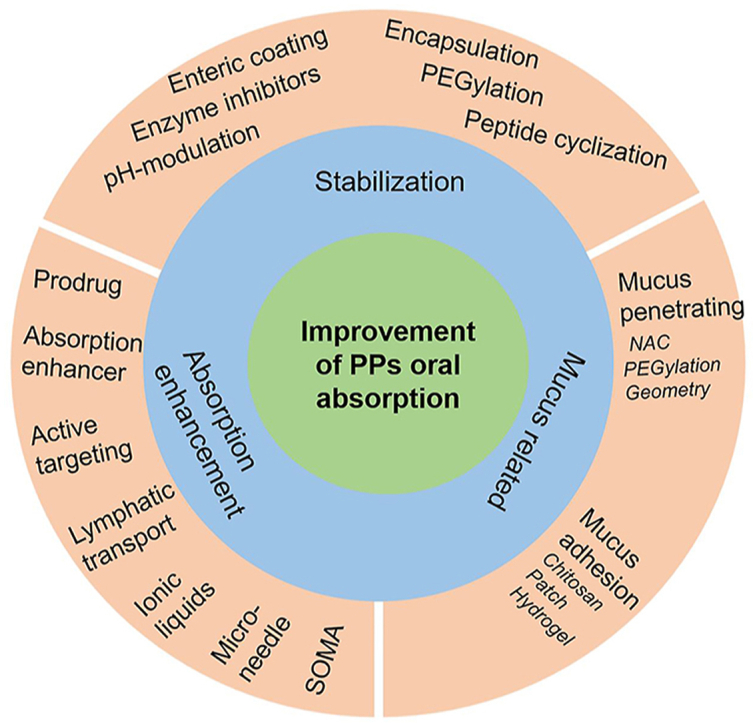
Proteins and peptides (PPs) have gradually become more attractive therapeutic molecules than small molecular drugs due to their high selectivity and efficacy, but fewer side effects. Owing to the poor stability and limited permeability through gastrointestinal (GI) tract and epithelia, the therapeutic PPs are usually administered by parenteral route. Given the big demand for oral administration in clinical use, a variety of researches focused on developing new technologies to overcome GI barriers of PPs, such as enteric coating, enzyme inhibitors, permeation enhancers, nanoparticles, as well as intestinal microdevices. Some new technologies have been developed under clinical trials and even on the market. This review summarizes the history, the physiological barriers and the overcoming approaches, current clinical and preclinical technologies, and future prospects of oral delivery of PPs.
Graphical abstract
Related collections
Most cited references371
- Record: found
- Abstract: found
- Article: not found
Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings.
- Record: found
- Abstract: found
- Article: not found
Mucus-penetrating nanoparticles for drug and gene delivery to mucosal tissues.
- Record: found
- Abstract: found
- Article: not found