- Record: found
- Abstract: found
- Article: found
Fermentative Foods: Microbiology, Biochemistry, Potential Human Health Benefits and Public Health Issues
Read this article at
Abstract
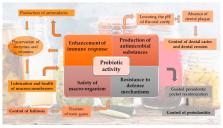
Fermented foods identify cultures and civilizations. History, climate and the particulars of local production of raw materials have urged humanity to exploit various pathways of fermentation to produce a wide variety of traditional edible products which represent adaptations to specific conditions. Nowadays, industrial-scale production has flooded the markets with ferments. According to recent estimates, the current size of the global market of fermented foods is in the vicinity of USD 30 billion, with increasing trends. Modern challenges include tailor-made fermented foods for people with special dietary needs, such as patients suffering from Crohn’s disease or other ailments. Another major challenge concerns the safety of artisan fermented products, an issue that could be tackled with the aid of molecular biology and concerns not only the presence of pathogens but also the foodborne microbial resistance. The basis of all these is, of course, the microbiome, an aggregation of different species of bacteria and yeasts that thrives on the carbohydrates of the raw materials. In this review, the microbiology of fermented foods is discussed with a special reference to groups of products and to specific products indicative of the diversity that a fermentation process can take. Their impact is also discussed with emphasis on health and oral health status. From Hippocrates until modern approaches to disease therapy, diet was thought to be of the most important factors for health stability of the human natural microbiome. After all, to quote Pasteur, “Gentlemen, the microbes will have the last word for human health.” In that sense, it is the microbiomes of fermented foods that will acquire a leading role in future nutrition and therapeutics.
Related collections
Most cited references227
- Record: found
- Abstract: found
- Article: not found
Origins and evolution of antibiotic resistance.
- Record: found
- Abstract: found
- Article: not found