- Record: found
- Abstract: found
- Article: found
Preclinical Evaluation of the Stability, Safety, and Efficacy of CD101, a Novel Echinocandin
Read this article at
Abstract
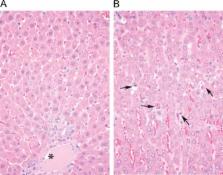
Fungal infections pose a significant public health burden with high morbidity and mortality. CD101 is a novel echinocandin under development for the treatment and prevention of systemic Candida infections. Preclinical studies were conducted to evaluate the metabolic stability, plasma protein binding, pharmacokinetics, toxicity, and efficacy of CD101 at various dose levels. CD101 was stable to biotransformation in rat, monkey, and human liver microsomes and rat, monkey, dog, and human hepatocytes. In vitro studies suggest minimal interaction with recombinant cytochrome P450 enzymes (50% inhibitory concentrations [IC 50s] of >10 μM). Similar to anidulafungin, CD101 bound avidly (>98%) to human, mouse, rat, and primate plasma proteins. In a 2-week repeat-dose comparison study, CD101 was well tolerated in rats (no effects on body weight, hematology, coagulation, or urinalysis). In contrast, administration of anidulafungin (at comparable exposure levels) resulted in reduced body weight, decreases in red blood cell, hemoglobin, hematocrit, mean cell volume, mean corpuscular hemoglobin, platelet, and reticulocyte counts, increases in neutrophil and eosinophil counts, polychromasia, and decreased activated partial thromboplastin time. Elevated plasma transaminases, total bilirubin, cholesterol, and globulin, dark and enlarged spleens, and single-cell hepatocyte necrosis were also observed for anidulafungin but not CD101. Hepatotoxicity may be due to the inherent chemical lability of anidulafungin generating potentially reactive intermediates. A glutathione trapping experiment confirmed the formation of a reactive species from anidulafungin, whereas CD101 did not exhibit instability or reactive intermediates. CD101 showed antifungal activity against Candida and Aspergillus infections in neutropenic mice. These preclinical studies demonstrated that CD101 is chemically and metabolically stable, well tolerated with no hepatotoxicity, and efficacious as an antifungal agent.
Related collections
Most cited references13
- Record: found
- Abstract: found
- Article: not found
Executive Summary: Clinical Practice Guideline for the Management of Candidiasis: 2016 Update by the Infectious Diseases Society of America.
- Record: found
- Abstract: found
- Article: not found
