- Record: found
- Abstract: found
- Article: found
LINC00511 is associated with the malignant status and promotes cell proliferation and motility in cervical cancer

Read this article at
Abstract
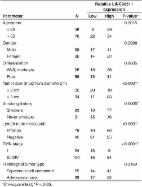
LINC00511 is a newly identified lncRNA that is up-regulated in many types of human cancers and may serve as an oncogenic lncRNA. However, there was no report about the role of LINC00511 in cervical cancer. Therefore, we investigated the clinical value of LINC00511 in cervical cancer patients via analyzing the correlation between LINC00511 expression and clinicopathological features. Moreover, we performed loss-of-function study to estimate the effect of LINC00511 on cervical cancer cell proliferation, migration, and invasion. In our study, we found LINC00511 expression levels were increased in cervical cancer tissues and cell lines compared with adjacent normal tissues and normal cervical epithelial cell line, respectively. High LINC00511 expression was correlated with advanced clinical stage, large tumor size, histological type of adenocarcinoma, and present lymph node metastasis, distant metastasis, and poor overall survival in cervical cancer patients. The in vitro studies indicated that knockdown of LINC00511 inhibited cervical cancer cell proliferation, migration, and invasion. In conclusion, LINC00511 acts as oncogenic lncRNA in cervical cancer, and may be a novel biomarker and potential therapeutic target for cervical cancer patients.
Related collections
Most cited references21

- Record: found
- Abstract: found
- Article: found
Long noncoding RNA LINC00511 contributes to breast cancer tumourigenesis and stemness by inducing the miR-185-3p/E2F1/Nanog axis
- Record: found
- Abstract: found
- Article: found
Long Intergenic Noncoding RNA 00511 Acts as an Oncogene in Non–small-cell Lung Cancer by Binding to EZH2 and Suppressing p57

- Record: found
- Abstract: found
- Article: found