- Record: found
- Abstract: found
- Article: not found
Integrating Brain Implants With Local and Distributed Computing Devices: A Next Generation Epilepsy Management System

Read this article at
Abstract
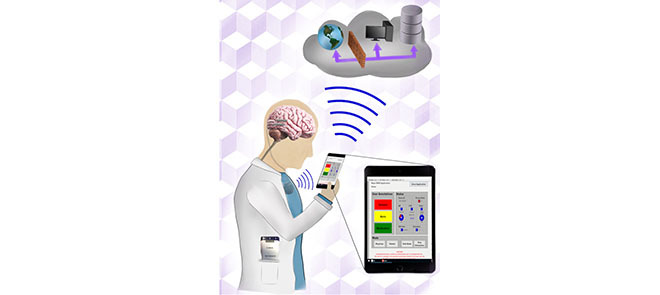
Brain stimulation has emerged as an effective treatment for a wide range of neurological and psychiatric diseases. Parkinson’s disease, epilepsy, and essential tremor have FDA indications for electrical brain stimulation using intracranially implanted electrodes. Interfacing implantable brain devices with local and cloud computing resources have the potential to improve electrical stimulation efficacy, disease tracking, and management. Epilepsy, in particular, is a neurological disease that might benefit from the integration of brain implants with off-the-body computing for tracking disease and therapy. Recent clinical trials have demonstrated seizure forecasting, seizure detection, and therapeutic electrical stimulation in patients with drug-resistant focal epilepsy. In this paper, we describe a next-generation epilepsy management system that integrates local handheld and cloud-computing resources wirelessly coupled to an implanted device with embedded payloads (sensors, intracranial EEG telemetry, electrical stimulation, classifiers, and control policy implementation). The handheld device and cloud computing resources can provide a seamless interface between patients and physicians, and realtime intracranial EEG can be used to classify brain state (wake/sleep, preseizure, and seizure), implement control policies for electrical stimulation, and track patient health. This system creates a flexible platform in which low demand analytics requiring fast response times are embedded in the implanted device and more complex algorithms are implemented in offthebody local and distributed cloud computing environments. The system enables tracking and management of epileptic neural networks operating over time scales ranging from milliseconds to months.
Abstract
Brain Implants integrated with Local and Distributed Computing Devices provide a seamless interface between patients and physicians, and real-time intracranial EEG can be used to classify brain state (wake/sleep, pre-seizure, seizure), implement control policies for electrical stimulation, and track patient health. The system enables tracking and management of epileptic neural networks operating over time scales ranging from milliseconds to months.
Related collections
Most cited references41
- Record: found
- Abstract: found
- Article: not found
Prediction of seizure likelihood with a long-term, implanted seizure advisory system in patients with drug-resistant epilepsy: a first-in-man study.
- Record: found
- Abstract: found
- Article: not found
A phase I trial of deep brain stimulation of memory circuits in Alzheimer's disease.
- Record: found
- Abstract: found
- Article: not found
