- Record: found
- Abstract: found
- Article: found
Pragmatic trials of pain therapies: a systematic review of methods
Read this article at
Abstract
Supplemental Digital Content is Available in the Text.
Abstract
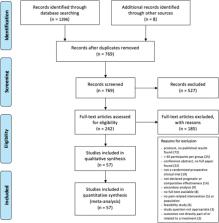
Pragmatic randomised clinical trials aim to directly inform clinical or health policy decision making. Here, we systematically review methods and design of pragmatic trials of pain therapies to examine methods, identify common challenges, and areas for improvement. Seven databases were searched for pragmatic randomised controlled clinical trials that assessed pain treatment in a clinical population of adults reporting pain. All screening steps and data extractions were performed twice. Data were synthesised descriptively, and correlation analyses between prespecified trial features and PRECIS-2 (PRagmatic–Explanatory Continuum Indicator Summary 2) ratings and attrition were performed. Protocol registration: PROSPERO-ID CRD42020178954. Of 57 included trials, only 21% assessed pharmacological interventions, the remainder physical, surgical, psychological, or self-management pain therapies. Three-quarters of the trials were comparative effectiveness designs, often conducted in multiple centres (median: 5; Q1/3: 1, 9.25) and with a median sample size of 234 patients at randomization (Q1/3: 135.5; 363.5). Although most trials recruited patients with chronic pain, reporting of pain duration was poor and not well described. Reporting was comprehensive for most general items, while often deficient for specific pragmatic aspects. Average ratings for pragmatism were highest for treatment adherence flexibility and clinical relevance of outcome measures. They were lowest for patient recruitment methods and extent of follow-up measurements and appointments. Current practice in pragmatic trials of pain treatments can be improved in areas such as patient recruitment and reporting of methods, analysis, and interpretation of data. These improvements will facilitate translatability to other real-world settings—the purpose of pragmatic trials.
Related collections
Most cited references130
- Record: found
- Abstract: found
- Article: found
Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement
- Record: found
- Abstract: not found
- Article: not found
RoB 2: a revised tool for assessing risk of bias in randomised trials
- Record: found
- Abstract: found
- Article: not found