- Record: found
- Abstract: found
- Article: found
The Impact of Pre-existing Immunity on the Non-clinical Pharmacodynamics of AAV5-Based Gene Therapy
Read this article at
Abstract
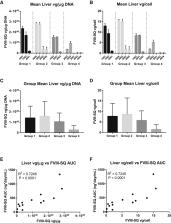
Adeno-associated virus (AAV)-based vectors are widely used for gene therapy, but the effect of pre-existing antibodies resulting from exposure to wild-type AAV is unclear. In addition, other poorly defined plasma factors could inhibit AAV vector transduction where antibodies are not detected. To better define the relationship between various forms of pre-existing AAV immunity and gene transfer, we studied valoctocogene roxaparvovec (BMN 270) in cynomolgus monkeys with varying pre-dose levels of neutralizing anti-AAV antibodies and non-antibody transduction inhibitors. BMN 270 is an AAV5-based vector for treating hemophilia A that encodes human B domain-deleted factor VIII (FVIII-SQ). After infusion of BMN 270 (6.0 × 10 13 vg/kg) into animals with pre-existing anti-AAV5 antibodies, there was a mean decrease in maximal FVIII-SQ plasma concentration (C max) and AUC of 74.8% and 66.9%, respectively, compared with non-immune control animals, and vector genomes in the liver were reduced. In contrast, animals with only non-antibody transduction inhibitors showed FVIII-SQ plasma concentrations and liver vector copies comparable with those of controls. These results demonstrate that animals without AAV5 antibodies are likely responders to AAV5 gene therapy, regardless of other inhibiting plasma factors. The biological threshold for tolerable AAV5 antibody levels varied between individual animals and should be evaluated further in clinical studies.
Related collections
Most cited references26
- Record: found
- Abstract: found
- Article: not found
Clades of Adeno-associated viruses are widely disseminated in human tissues.
- Record: found
- Abstract: found
- Article: not found
Therapeutic levels of FVIII following a single peripheral vein administration of rAAV vector encoding a novel human factor VIII variant.
- Record: found
- Abstract: found
- Article: not found