- Record: found
- Abstract: found
- Article: found
Immune Responses to Viral Gene Therapy Vectors
Read this article at
Abstract
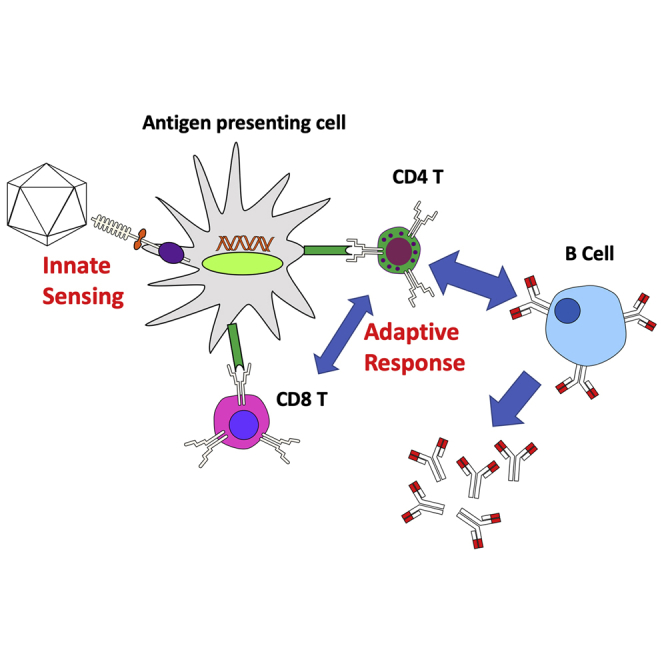
Several viral vector-based gene therapy drugs have now received marketing approval. A much larger number of additional viral vectors are in various stages of clinical trials for the treatment of genetic and acquired diseases, with many more in pre-clinical testing. Efficiency of gene transfer and ability to provide long-term therapy make these vector systems very attractive. In fact, viral vector gene therapy has been able to treat or even cure diseases for which there had been no or only suboptimal treatments. However, innate and adaptive immune responses to these vectors and their transgene products constitute substantial hurdles to clinical development and wider use in patients. This review provides an overview of the type of immune responses that have been documented in animal models and in humans who received gene transfer with one of three widely tested vector systems, namely adenoviral, lentiviral, or adeno-associated viral vectors. Particular emphasis is given to mechanisms leading to immune responses, efforts to reduce vector immunogenicity, and potential solutions to the problems. At the same time, we point out gaps in our knowledge that should to be filled and problems that need to be addressed going forward.
Graphical Abstract
Abstract
Viral vectors are successfully used in human gene therapy. However, immune responses complicate their use, ranging from early innate responses and immunotoxicity to subsequent adaptive immune responses to the vector or transgene product. This article reviews immune response mechanisms against adenoviral, adeno-associated viral, and lentiviral vectors.
Related collections
Most cited references139

- Record: found
- Abstract: found
- Article: not found
Successful transduction of liver in hemophilia by AAV-Factor IX and limitations imposed by the host immune response.
- Record: found
- Abstract: found
- Article: found