- Record: found
- Abstract: found
- Article: found
Biomass-fuelled improved cookstove intervention to prevent household air pollution in Northwest Ethiopia: a cluster randomized controlled trial
Read this article at
Abstract
Background
Household air pollution from biomass fuels burning in traditional cookstoves currently appeared as one of the most serious threats to public health with a recent burden estimate of 2.6 million premature deaths every year worldwide, ranking highest among environmental risk factors and one of the major risk factors of any type globally. Improved cookstove interventions have been widely practiced as potential solutions. However, studies on the effect of improved cookstove interventions are limited and heterogeneous which suggested the need for further research.
Methods
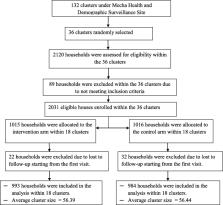
A cluster randomized controlled trial study was conducted to assess the effect of biomass-fuelled improved cookstove intervention on the concentration of household air pollution compared with the continuation of an open burning traditional cookstove. A total of 36 clusters were randomly allocated to both arms at a 1:1 ratio, and improved cookstove intervention was delivered to all households allocated into the treatment arm. All households in the included clusters were biomass fuel users and relatively homogenous in terms of basic socio-demographic and cooking-related characteristics. Household air pollution was determined by measuring the concentration of indoor fine particulate, and the effect of the intervention was estimated using the Generalized Estimating Equation.
Results
A total of 2031 household was enrolled in the study across 36 randomly selected clusters in both arms, among which data were obtained from a total of 1977 households for at least one follow-up visit which establishes the intention-to-treat population dataset for analysis. The improved cookstove intervention significantly reduces the concentration of household air pollution by about 343 μg/m 3 ( Ḃ = − 343, 95% CI − 350, − 336) compared to the traditional cookstove method. The overall reduction was found to be about 46% from the baseline value of 859 (95% CI 837–881) to 465 (95% CI 458–472) in the intervention arm compared to only about 5% reduction from 850 (95% CI 828–872) to 805 (95% CI 794–817) in the control arm.
Conclusions
The biomass-fuelled improved cookstove intervention significantly reduces the concentration of household air pollution compared to the traditional method. This suggests that the implementation of these cookstove technologies may be necessary to achieve household air pollution exposure reductions.
Trial registration
The trial project was retrospectively registered on August 2, 2018, at the clinical trials.gov registry database ( https://clinicaltrials.gov/) with the NCT03612362 registration identifier number.
Related collections
Most cited references66
- Record: found
- Abstract: found
- Article: not found
A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010
- Record: found
- Abstract: found
- Article: found
Better reporting of interventions: template for intervention description and replication (TIDieR) checklist and guide

- Record: found
- Abstract: found
- Article: found
Global, regional, and national comparative risk assessment of 84 behavioural, environmental and occupational, and metabolic risks or clusters of risks for 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017
Author and article information
Comments
Comment on this article
 Smart Citations
Smart CitationsSee how this article has been cited at scite.ai
scite shows how a scientific paper has been cited by providing the context of the citation, a classification describing whether it supports, mentions, or contrasts the cited claim, and a label indicating in which section the citation was made.