- Record: found
- Abstract: found
- Article: found
Topography and distribution of adenosine A 2A and dopamine D 2 receptors in the human Subthalamic Nucleus
Read this article at
Abstract
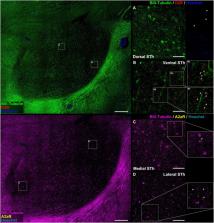
The human Subthalamic Nucleus (STh) is a diencephalic lens-shaped structure located ventrally to the thalamus and functionally implicated in the basal ganglia circuits. Despite recent efforts to characterize the neurochemical and functional anatomy of the STh, little to no information is available concerning the expression and distribution of receptors belonging to the dopaminergic and purinergic system in the human STh. Both systems are consistently implicated in basal ganglia physiology and pathology, especially in Parkinson’s Disease, and represent important targets for the pharmacological treatment of movement disorders. Here, we investigate the topography and distribution of A 2A adenosine and D 2 dopamine receptors in the human basal ganglia and subthalamic nucleus. Our findings indicate a peculiar topographical distribution of the two receptors throughout the subthalamic nucleus, while colocalization between the receptors opens the possibility for the presence of A 2AR- D 2R heterodimers within the dorsal and medial aspects of the structure. However, further investigation is required to confirm these findings.
Related collections
Most cited references43
- Record: found
- Abstract: found
- Article: not found
Adenosine-dopamine receptor-receptor interactions as an integrative mechanism in the basal ganglia.
- Record: found
- Abstract: found
- Article: not found
Adenosine A2A receptors and basal ganglia physiology.
- Record: found
- Abstract: found
- Article: not found