- Record: found
- Abstract: found
- Article: found
Three new ent-abietane diterpenoids from the roots of Euphorbia fischeriana and their cytotoxicity in human tumor cell lines
Read this article at
Abstract
Abstract
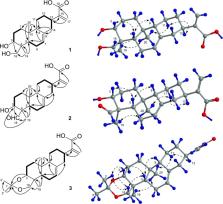
Three new ent-abietane diterpenoids, termed fischerianoids A–C ( 1– 3), were isolated and identified from the ethyl acetate extracts of roots of the medicinally valuable plant Euphorbia fischeriana. The planar and relative structures of 1– 3 were established via high-resolution electrospray ionisation mass spectrometry and one- and two-dimensional nuclear magnetic resonance spectroscopic analyses, and the absolute configuration of 1 was further established via X-ray crystallography experiment. Compounds 1– 3 showed selective inhibitory potency against certain human tumor cell lines with IC 50 values ranging from 8.50 ± 0.13 to 35.52 ± 0.08 μM.
Related collections
Most cited references11
- Record: found
- Abstract: found
- Article: not found
Absolute structure and absolute configuration
- Record: found
- Abstract: not found
- Article: not found
SHELXL: high-resolution refinement.
- Record: found
- Abstract: found
- Article: not found
