- Record: found
- Abstract: found
- Article: found
Multiple forms of working memory emerge from synapse–astrocyte interactions in a neuron–glia network model
Read this article at
Significance
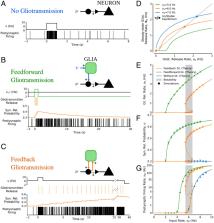
The biophysical underpinnings of working memory are of paramount interest in modern neuroscience. Working memory could be encoded by diverse patterns of neural activity, ranging from persistent firing of neuronal populations to more dynamic patterns of network activity. Silent mechanisms whereby working memory is maintained by synaptic variables have also been suggested. Multiple models exist for these mechanisms but only consider neurons, ignoring glia. We propose that glia could underpin working memory, introducing models of cortical neuron–glial networks where synapse–glia signaling could account for firing and silent working memory encoding. Our theoretical arguments can explain emerging accounts of the variegated nature of working memory encoding and the possible contribution of glia to it.
Abstract
Persistent activity in populations of neurons, time-varying activity across a neural population, or activity-silent mechanisms carried out by hidden internal states of the neural population have been proposed as different mechanisms of working memory (WM). Whether these mechanisms could be mutually exclusive or occur in the same neuronal circuit remains, however, elusive, and so do their biophysical underpinnings. While WM is traditionally regarded to depend purely on neuronal mechanisms, cortical networks also include astrocytes that can modulate neural activity. We propose and investigate a network model that includes both neurons and glia and show that glia–synapse interactions can lead to multiple stable states of synaptic transmission. Depending on parameters, these interactions can lead in turn to distinct patterns of network activity that can serve as substrates for WM.
Related collections
Most cited references65
- Record: found
- Abstract: found
- Article: not found
Diversity of astrocyte functions and phenotypes in neural circuits.
- Record: found
- Abstract: found
- Article: not found
Dynamics of sparsely connected networks of excitatory and inhibitory spiking neurons.
- Record: found
- Abstract: found
- Article: not found