- Record: found
- Abstract: found
- Article: found
Cytokine-Induced Modulation of Colorectal Cancer
Read this article at
Abstract
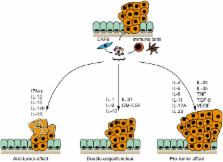
The emergence of novel immunomodulatory cancer therapies over the last decade, above all immune checkpoint blockade, has significantly advanced tumor treatment. For colorectal cancer (CRC), a novel scoring system based on the immune cell infiltration in tumors has greatly improved disease prognostic evaluation and guidance to more specific therapy. These findings underline the relevance of tumor immunology in the future handling and therapeutic approach of malignant disease. Inflammation can either promote or suppress CRC pathogenesis and inflammatory mediators, mainly cytokines, critically determine the pro- or anti-tumorigenic signals within the tumor environment. Here, we review the current knowledge on the cytokines known to be critically involved in CRC development and illustrate their mechanisms of action. We also highlight similarities and differences between CRC patients and murine models of CRC and point out cytokines with an ambivalent role for intestinal cancer. We also identify some of the future challenges in the field that should be addressed for the development of more effective immunomodulatory therapies.
Related collections
Most cited references205
- Record: found
- Abstract: found
- Article: not found
The inhibitory cytokine IL-35 contributes to regulatory T-cell function.
- Record: found
- Abstract: found
- Article: not found
Myeloid-derived suppressor cells: linking inflammation and cancer.
- Record: found
- Abstract: found
- Article: not found