- Record: found
- Abstract: found
- Article: found
A randomized double-blind placebo-controlled trial of probiotics in post-surgical colorectal cancer
Read this article at
Abstract
Background
Our study aimed to determine the effect of probiotic consumption containing six viable microorganisms of 30 × 10 10 cfu Lactobacillus and Bifidobacteria strains for six months on clinical outcomes and inflammatory cytokines (TNF-α, IFN-γ, IL-6, IL-10, IL-12, IL-17A, IL-17C and IL-22) in patients with colorectal cancer.
Methods
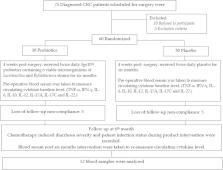
Fifty-two patients with colorectal cancer were randomized at four weeks after surgery to receive either a placebo ( n = 25) or 30 billion colony-forming unit (CFU) of a mixture of six viable strains including 107 mg of Lactobacillus acidophilus BCMC® 12,130, Lactobacillus lactis BCMC® 12,451, Lactobacillus casei subsp BCMC® 12,313, Bifidobacterium longum BCMC® 02120, Bifidobacterium bifidum BCMC® 02290 and Bifidobacterium infantis BCMC® 02129 ( n = 27). Patients were instructed to take the product orally twice daily for six months. Infection status, diarrhea or hospital admission were recorded throughout the study. Blood was taken pre- and post-intervention to measure TNF-α, IFN-γ, IL-6, IL-10, IL-12, IL-17A, IL-17C and IL-22 using ELISA multiplex kit.
Results
The majority of cases (~ 70%) were in Duke’s C colorectal cancer for both groups. No surgical infection occurred and no antibiotics were required. Chemotherapy induced diarrhea was observed in both groups. Significant reduction in the level of pro-inflammatory cytokine, TNF-α, IL-6, IL-10, IL-12, IL-17A, IL-17C and IL-22 were observed in CRC patients who received probiotics as compared to pre-treatment level ( P < 0.05). However, there was no significant difference in the IFN-γ in both groups.
Conclusions
We have shown that probiotics containing six viable microorganisms of Lactobacillus and Bifidobacteria strains are safe to be consumed at four weeks after surgery in colorectal cancer patients and have reduced pro-inflammatory cytokines (except for IFN-gamma). Probiotic may modify intestinal microenvironment resulting in a decline in pro-inflammatory cytokines.
Related collections
Most cited references24
- Record: found
- Abstract: found
- Article: not found
Probiotics and antibodies to TNF inhibit inflammatory activity and improve nonalcoholic fatty liver disease.
- Record: found
- Abstract: found
- Article: not found
Probiotic bacteria enhance murine and human intestinal epithelial barrier function.
- Record: found
- Abstract: found
- Article: not found