- Record: found
- Abstract: found
- Article: found
SNHG12 inhibits oxygen-glucose deprivation-induced neuronal apoptosis via the miR-181a-5p/NEGR1 axis
Read this article at
Abstract
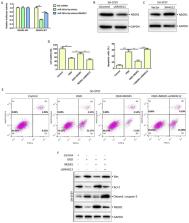
Emerging evidence has indicated that long non-coding RNAs (lncRNAs) are closely associated with the pathogenesis of ischemic stroke. It has been reported that small nucleolar RNA host gene 12 (SNHG12) serves a critical role in ischemic stroke by acting as a competitive endogenous RNA (ceRNA). SNHG12 competes with various microRNAs (miRs) to regulate RNA transcription of specific targets. However, the effect of SNHG12 on oxygen-glucose deprivation (OGD)-induced neuronal apoptosis has rarely been reported. The present study demonstrated that SNHG12 expression was downregulated in OGD-injured SH-SY5Y cells. Furthermore, miR-181a-5p was reported as a target of SNHG12 and was negatively regulated by SNHG12. Moreover, NEGR1 was a target of miR-181a-5p, which functions as a negative regulator of NEGR1 in OGD-induced neuronal apoptosis. In summary, the results strongly confirmed the hypothesis that SNHG12 functions as a ceRNA for miR-181a-5p and regulates the expression of NEGR1 thus inhibiting OGD-induced apoptosis of SH-SY5Y cells. Neuronal apoptosis aggravates brain damage during ischemic stroke, indicating that the activation of SNHG12 and NEGR1 expression and inhibition of miR-181a-5p may be a novel strategy for the clinical treatment of ischemic stroke.
Related collections
Most cited references28
- Record: found
- Abstract: found
- Article: not found
miR-181 regulates GRP78 and influences outcome from cerebral ischemia in vitro and in vivo.
- Record: found
- Abstract: found
- Article: found
Circular RNAs: A novel type of non-coding RNA and their potential implications in antiviral immunity
- Record: found
- Abstract: found
- Article: not found