- Record: found
- Abstract: found
- Article: found
A small animal model of chronic hepatitis E infection using immunocompromised rats

Read this article at
Abstract
Background & Aims
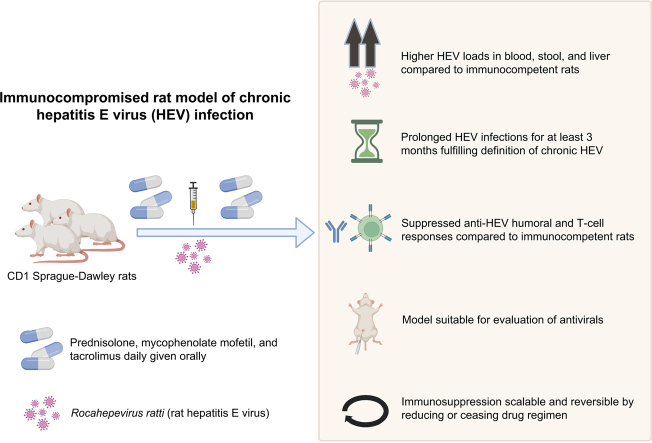
HEV variants such as swine genotypes within Paslahepevirus species balayani (HEV-A) and rat HEV ( Rocahepevirus ratti; HEV-C1) cause chronic hepatitis E in immunocompromised individuals. There are few reliable and accessible small animal models that accurately reflect chronic HEV infection. We aimed to develop an immunocompromised rat model of chronic hepatitis E infection.
Methods
In this animal model infection study, rats were immunosuppressed with a drug combination (prednisolone, tacrolimus, and mycophenolate mofetil) commonly taken by transplant recipients. Rats were challenged with human- and rat-derived HEV-C1 strains or a human-derived HEV-A strain. Viral load, liver function, liver histology, humoural, and cellular immune responses were monitored.
Results
A high-dose (HD) immunosuppressive regimen consistently prolonged human- and rat-derived HEV-C1 infection in rats (up to 12 weeks post infection) compared with transient infections in low-dose (LD) immunosuppressant-treated and immunocompetent (IC) rats. Mean HEV-C1 viral loads in stool, serum, and liver tissue were higher in HD regimen-treated rats than in LD or IC rats ( p <0.05). Alanine aminotransferase elevation was observed in chronically infected rats, which was consistent with histological hepatitis and HEV-C1 antigen expression in liver tissue. None (0/6) of the HD regimen-treated, 5/6 LD regimen-treated, and 6/6 IC rats developed antibodies to HEV-C1 in species-specific immunoblots. Reversal of immunosuppression was associated with clearance of viraemia and restoration of HEV-C1-specific humoural and cellular immune responses in HD regimen-treated rats, mimicking patterns in treated patients with chronic hepatitis E. Viral load suppression was observed with i.p. ribavirin treatment. HD regimen-treated rats remained unsusceptible to HEV-A infection.
Conclusions
We developed a scalable immunosuppressed rat model of chronic hepatitis E that closely mimics this infection phenotype in transplant recipients.
Lay summary
Convenient small animal models are required for the study of chronic hepatitis E in humans. We developed an animal model of chronic hepatitis E by suppressing immune responses of rats with drugs commonly taken by humans as organ transplant rejection prophylaxis. This model closely mimicked features of chronic hepatitis E in humans.
Graphical abstract
Highlights
-
•
Chronic HEV infection is challenging to model with small animals.
-
•
Rats can be immunocompromised by transplant rejection drugs taken by patients.
-
•
This model supports chronic rat HEV infection robustly and consistently.
-
•
Immunosuppression in this model is scalable, reversible, and responsive to ribavirin.
Related collections
Most cited references39
- Record: found
- Abstract: found
- Article: not found
EASL Clinical Practice Guidelines on hepatitis E virus infection
- Record: found
- Abstract: found
- Article: not found
Hepatitis E virus and chronic hepatitis in organ-transplant recipients.
- Record: found
- Abstract: found
- Article: not found