- Record: found
- Abstract: found
- Article: found
PINK1 drives Parkin self-association and HECT-like E3 activity upstream of mitochondrial binding
Read this article at
Abstract
PINK1 activates the HECT-like E3 ubiquitin ligase activity and self-association of Parkin upstream of its translocation to mitochondria and induction of mitophagy.
Abstract
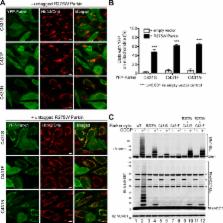
Genetic studies indicate that the mitochondrial kinase PINK1 and the RING-between-RING E3 ubiquitin ligase Parkin function in the same pathway. In concurrence, mechanistic studies show that PINK1 can recruit Parkin from the cytosol to the mitochondria, increase the ubiquitination activity of Parkin, and induce Parkin-mediated mitophagy. Here, we used a cell-free assay to recapitulate PINK1-dependent activation of Parkin ubiquitination of a validated mitochondrial substrate, mitofusin 1. We show that PINK1 activated the formation of a Parkin–ubiquitin thioester intermediate, a hallmark of HECT E3 ligases, both in vitro and in vivo. Parkin HECT-like ubiquitin ligase activity was essential for PINK1-mediated Parkin translocation to mitochondria and mitophagy. Using an inactive Parkin mutant, we found that PINK1 stimulated Parkin self-association and complex formation upstream of mitochondrial translocation. Self-association occurred independent of ubiquitination activity through the RING-between-RING domain, providing mechanistic insight into how PINK1 activates Parkin.