- Record: found
- Abstract: found
- Article: found
Potassium Uptake Systems in Staphylococcus aureus: New Stories about Ancient Systems
Read this article at
ABSTRACT
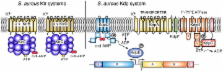
Staphylococcus aureus is a hardy organism that can survive high-salt conditions better than many other bacteria. This characteristic is thought to help S. aureus survive in the nares and on the skin of the human host and is used to selectively propagate and identify Staphylococcus species. However, the mechanism that allows S. aureus to tolerate such high-salt conditions is not well understood. A recent study in mBio by A. Price-Whelan et al. [mBio 4(4):e00407-13, 2013, doi:10.1128/mBio.00407-13] highlights the importance of potassium uptake in this process. This commentary provides a perspective of the study by Price-Whelan et al. as well as other recently reported work on potassium uptake and transport systems in S. aureus.
Related collections
Most cited references18
- Record: found
- Abstract: found
- Article: not found
Cyclic di-AMP: another second messenger enters the fray.
- Record: found
- Abstract: found
- Article: not found
The roles and regulation of potassium in bacteria.
- Record: found
- Abstract: found
- Article: not found