- Record: found
- Abstract: found
- Article: found
Expression of endothelia and lymphocyte adhesion molecules in bronchus-associated lymphoid tissue (BALT) in adult human lung
Read this article at
Abstract
Background
Bronchus-associated lymphoid tissue (BALT) is the secondary lymphoid tissue in bronchial mucosa and is involved in the development of bronchopulmonary immune responses. Although migration of lymphocytes from blood vessels into secondary lymphoid tissues is critical for the development of appropriate adaptive immunity, the endothelia and lymphocyte adhesion molecules that recruit specific subsets of lymphocytes into human BALT are not known. The aim of this study was to determine which adhesion molecules are expressed on lymphocytes and high endothelial venules (HEVs) in human BALT.
Methods
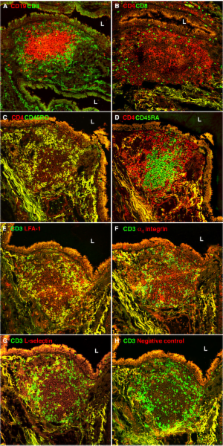
We immunostained frozen sections of BALT from lobectomy specimens from 17 patients with lung carcinoma with a panel of monoclonal antibodies to endothelia and lymphocyte adhesion molecules.
Results
Sections of BALT showed B cell follicles surrounded by T cells. Most BALT CD4 + T cells had a CD45RO + memory phenotype. Almost all BALT B cells expressed α 4 integrin and L-selectin. In contrast, 43% of BALT T cells expressed α 4 integrin and 20% of BALT T cells expressed L-selectin. Almost all BALT lymphocytes expressed LFA-1. HEVs, which support the migration of lymphocytes from the bloodstream into secondary lymphoid tissues, were prominent in BALT. All HEVs expressed peripheral node addressin, most HEVs expressed vascular cell adhesion molecule-1, and no HEVs expressed mucosal addressin cell adhesion molecule-1.
Related collections
Most cited references48
- Record: found
- Abstract: not found
- Article: not found
Chemokines and cell migration in secondary lymphoid organs.
- Record: found
- Abstract: found
- Article: not found