- Record: found
- Abstract: found
- Article: not found
Pitx2 promotes heart repair by activating the antioxidant response after cardiac injury
Read this article at
Summary
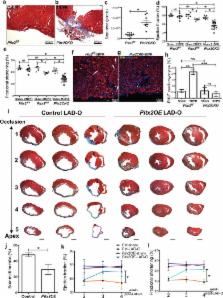
Myocardial infarction results in compromised myocardial function with heart failure due to insufficient cardiomyocyte self-renewal 1 . Unlike lower vertebrates, mammalian hearts only have a transient neonatal renewal capacity 2 . Reactivating primitive reparative ability in the mature heart requires knowledge of the mechanisms promoting early heart repair. By testing an established Hippo-deficient heart regeneration model for renewal promoting factors, we found that Pitx2 expression was induced in injured, Hippo-deficient ventricles. Pitx2-deficient neonatal hearts failed to repair after apex resection while Pitx2-gain-of-function in adult cardiomyocytes conferred reparative ability after myocardial infarction. Genomic analyses indicated that Pitx2 activated genes encoding electron transport chain components and reactive oxygen species scavengers. A subset of Pitx2 target genes was cooperatively regulated with the Hippo effector, Yap. Furthermore, Nrf2, a regulator of antioxidant response 3 , directly regulated Pitx2 expression and subcellular localization. Pitx2 mutant myocardium had elevated reactive oxygen species levels while antioxidant supplementation suppressed the Pitx2-loss-of-function phenotype. These findings reveal a genetic pathway, activated by tissue damage that is essential for cardiac repair.
Related collections
Most cited references13
- Record: found
- Abstract: found
- Article: not found
The oxygen-rich postnatal environment induces cardiomyocyte cell-cycle arrest through DNA damage response.
- Record: found
- Abstract: found
- Article: not found
Hippo signaling impedes adult heart regeneration.

- Record: found
- Abstract: found
- Article: found
