- Record: found
- Abstract: found
- Article: found
Activation of TLR3 Induces Osteogenic Responses in Human Aortic Valve Interstitial Cells through the NF-κB and ERK1/2 Pathways
Read this article at
Abstract
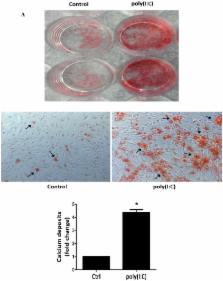
Calcific aortic valve disease (CAVD) is characterized by chronic inflammation and progressive calcification in valve leaflets. Aortic valve interstitial cells (AVICs) play a critical role in the pathogenesis of CAVD. Previous studies show that stimulation of Toll-like receptor (TLR) 2 or TLR4 in AVICs in vitro up-regulates the expression of osteogenic mediators. Double-stranded RNA (dsRNA) can activate pro-inflammatory signaling through TLR3, the NLRP3 inflammasome and RIG-I-like receptors. The objective of this study is to determine the effect of dsRNA on AVIC osteogenic activities and the mechanism of its action. Methods and results: AVICs isolated from normal human valves were exposed to polyinosinic-polycytidylic acid [poly(I:C)], a mimic of dsRNA. Treatment with poly(I:C) increased the production of bone morphogenetic protein-2 (BMP-2), transforming growth factor beta-1 (TGF-β1) and alkaline phosphatase (ALP), and resulted in calcium deposit formation. Poly(I:C) induced the phosphorylation of NF-κB and ERK1/2. Knockdown of TLR3 essentially abrogated NF-κB and ERK1/2 phosphorylation, and markedly reduced the effect of poly(I:C) on the production of BMP-2, TGF-β1 and ALP. Further, inhibition of either NF-κB or ERK1/2 markedly reduced the levels of BMP-2, TGF-β1 and ALP in cells exposed to poly(I:C). Conclusion: Poly(I:C) up-regulates the production of BMP-2, TGF-β1 and ALP, and promotes calcium deposit formation in human AVICs. The pro-osteogenic effect of poly(I:C) is mediated primarily by TLR3 and the NF-κB and ERK1/2 pathways. These findings suggest that dsRNA, when present in aortic valve tissue, may promote CAVD progression through up-regulation of AVIC osteogenic activities.
Related collections
Most cited references37
- Record: found
- Abstract: found
- Article: not found
TLR3: interferon induction by double-stranded RNA including poly(I:C).
- Record: found
- Abstract: found
- Article: not found
The emerging role of valve interstitial cell phenotypes in regulating heart valve pathobiology.
- Record: found
- Abstract: found
- Article: not found