- Record: found
- Abstract: found
- Article: found
Preservation solutions for attenuation of ischemia–reperfusion injury in vascularized composite allotransplantation

Read this article at
Abstract
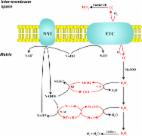
Vascularized composite allotransplantation represents the final level of the reconstructive ladder, offering treatment options for severe tissue loss and functional deficiencies. Vascularized composite allotransplantation is particularly susceptible to ischemia–reperfusion injury and requires preservation techniques when subjected to extended storage times prior to transplantation. While static cold storage functions to reduce ischemic damage and is widely employed in clinical settings, there exists no consensus on the ideal preservation solution for vascularized composite allotransplantation. This review aims to highlight current clinical and experimental advances in preservation solution development and their critical role in attenuating ischemia–reperfusion injury in the context of vascularized composite allotransplantation.
Related collections
Most cited references114
- Record: found
- Abstract: found
- Article: found
Reperfusion injury and reactive oxygen species: The evolution of a concept☆
- Record: found
- Abstract: found
- Article: not found