- Record: found
- Abstract: found
- Article: found
Folfirinox versus gemcitabine/nab-paclitaxel as first-line therapy in patients with metastatic pancreatic cancer: a comparative propensity score study
Read this article at
Abstract
Background:
Folfirinox (FFX) and gemcitabine/nab-paclitaxel (GN) are both standard first-line treatments in patients with metastatic pancreatic cancer (mPC). However, data comparing these two chemotherapeutic regimens and their sequential use remain scarce.
Methods:
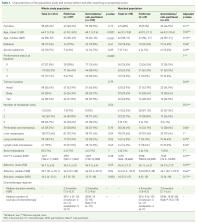
Data from two independent cohorts enrolling patients treated with FFX ( n = 107) or GN ( n = 109) were retrospectively pooled. Primary endpoint was overall survival (OS). Progression-free survival (PFS) was the secondary endpoint. A propensity score based on age, gender, performance status (PS), and presence of liver metastases was used to make groups comparable.
Results:
In the whole study population, OS was significantly higher in FFX (14 months; 95% CI: 10–21) than in GN groups (9 months; 95% CI: 8–12) before ( p = 0.008) and after ( p = 0.021) adjusting for age, number of metastatic sites, liver metastases, peritoneal carcinomatosis and CA19.9 level at baseline. PFS tends to be higher in FFX (6 months) than GN groups (5 months; p = 0.053). After matching ( n = 49/group), patients were comparable for all baseline characteristics including PS. In the matched population, there was a trend toward greater OS in patients treated with FFX (HR = 0.67; p = 0.097). However, survival in each group was not solely a result of the first-line regimen. The proportion of patients who were fit for GN after FFX failure (FFX–GN sequence) was higher (46.9%) than the reverse sequence (20.4%; p = 0.01), which suggests a higher feasibility for the FFX–GN sequence. Corresponding median OS were 19 months versus 9.5 months, respectively ( p = 0.094).
Related collections
Most cited references17
- Record: found
- Abstract: not found
- Article: not found
MatchIt: Nonparametric Preprocessing for Parametric Causal Inference
- Record: found
- Abstract: found
- Article: not found
