- Record: found
- Abstract: found
- Article: found
Gadd153 and NF-κB Crosstalk Regulates 27-Hydroxycholesterol-Induced Increase in BACE1 and β-Amyloid Production in Human Neuroblastoma SH-SY5Y Cells
Read this article at
Abstract
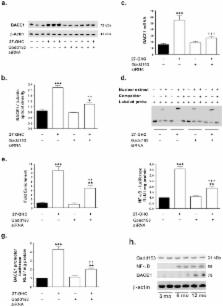
β-amyloid (Aβ) peptide, accumulation of which is a culprit for Alzheimer’s disease (AD), is derived from the initial cleavage of amyloid precursor protein by the aspartyl protease BACE1. Identification of cellular mechanisms that regulate BACE1 production is of high relevance to the search for potential disease-modifying therapies that inhibit BACE1 to reduce Aβ accumulation and AD progression. In the present study, we show that the cholesterol oxidation product 27-hydroxycholesterol (27-OHC) increases BACE1 and Aβ levels in human neuroblastoma SH-SY5Y cells. This increase in BACE1 involves a crosstalk between the two transcription factors NF-κB and the endoplasmic reticulum stress marker, the growth arrest and DNA damage induced gene-153 (gadd153, also called CHOP). We specifically show that 27-OHC induces a substantial increase in NF-κB binding to the BACE1 promoter and subsequent increase in BACE1 transcription and Aβ production. The NF-κB inhibitor, sc514, significantly attenuated the 27-OHC-induced increase in NF-κB-mediated BACE1 expression and Aβ genesis. We further show that the 27-OHC-induced NF-κB activation and increased NF-κB-mediated BACE1 expression is contingent on the increased activation of gadd153. Silencing gadd153 expression with siRNA alleviated the 27-OHC-induced increase in NF-κB activation, NF-κB binding to the BACE1 promoter, and subsequent increase in BACE1 transcription and Aβ production. We also show that increased levels of BACE1 in the triple transgenic mouse model for AD is preceded by gadd153 and NF-κB activation. In summary, our study demonstrates that gadd153 and NF-κB work in concert to regulate BACE1 expression. Agents that inhibit gadd153 activation and subsequent interaction with NF-κB might be promising targets to reduce BACE1 and Aβ overproduction and may ultimately serve as disease-modifying treatments for AD.
Related collections
Most cited references36
- Record: found
- Abstract: not found
- Article: not found
Active and Inactive Protein Kinases: Structural Basis for Regulation
- Record: found
- Abstract: found
- Article: not found
I kappa B: a specific inhibitor of the NF-kappa B transcription factor.
- Record: found
- Abstract: found
- Article: not found