- Record: found
- Abstract: found
- Article: found
The Giant HECT E3 Ubiquitin Ligase HERC1 Is Aberrantly Expressed in Myeloid Related Disorders and It Is a Novel BCR-ABL1 Binding Partner
Read this article at
Abstract
Simple Summary
The pathological role/s of the HERC family members has recently been initiated to be explored in few solid tumors and the assessment of their transcript amount reveals that they might act as effective prognostic factors. However, evidence concerning the non-solid tumors, and especially myeloid related neoplasms, is currently lacking. In the present article for the first time we provide original data for a clear and well-defined association between the gene expression level of a giant HERC E3 ubiquitin ligase family member, HERC1, and some myeloid related disorders, namely Acute Myeloid Leukemia, Myeloproliferative neoplasms and Chronic Myeloid Leukemia. Furthermore, our findings unveil that the HERC1 protein physically interacts, likely forming a very large supramolecular complex, and it is a putative BCR-ABL1 tyrosine kinase substrate. We hope that this work will contribute to the advance of our understanding of the roles played by the giant HERCs in myeloid related neoplasms.
Abstract
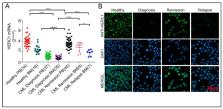
HERC E3 subfamily members are parts of the E3 ubiquitin ligases and key players for a wide range of cellular functions. Though the involvement of the Ubiquitin Proteasome System in blood disorders has been broadly studied, so far the role of large HERCs in this context remains unexplored. In the present study we examined the expression of the large HECT E3 Ubiquitin Ligase, HERC1, in blood disorders. Our findings revealed that HERC1 gene expression was severely downregulated both in acute and in chronic myelogenous leukemia at diagnosis, while it is restored after complete remission achievement. Instead, in Philadelphia the negative myeloproliferative neoplasm HERC1 level was peculiarly controlled, being very low in Primary Myelofibrosis and significantly upregulated in those Essential Thrombocytemia specimens harboring the mutation in the calreticulin gene. Remarkably, in CML cells HERC1 mRNA level was associated with the BCR-ABL1 kinase activity and the HERC1 protein physically interacted with BCR-ABL1. Furthermore, we found that HERC1 was directly tyrosine phosphorylated by the ABL kinase. Overall and for the first time, we provide original evidence on the potential tumor-suppressing or -promoting properties, depending on the context, of HERC1 in myeloid related blood disorders.
Related collections
Most cited references44
- Record: found
- Abstract: found
- Article: not found
Ubiquitination in disease pathogenesis and treatment.
- Record: found
- Abstract: found
- Article: not found