- Record: found
- Abstract: found
- Article: found
A Facile Synthesis of N-H- and N-Substituted Acridine-1,8-diones under Sonic Condition
research-article
31 December 2013
Read this article at
There is no author summary for this article yet. Authors can add summaries to their articles on ScienceOpen to make them more accessible to a non-specialist audience.
Abstract
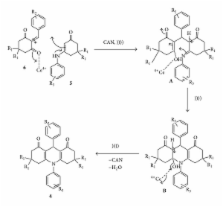
Synthesis of an assembly of structurally important N-H- and N-substituted acridine-1,8-diones by CAN (ceric ammonium nitrate) catalysed one-pot four-component reaction of electron-deficient and electron-rich aromatic aldehydes and aromatic amines or ammonium acetate and dimedone or cyclohexyl-1,3-diones at 26°C under sonic condition is reported. The method is clean and energy efficient as it uses a greener method and an eco-friendly catalyst.
Related collections
Most cited references35
- Record: found
- Abstract: not found
- Article: not found
Ultrasound in synthetic organic chemistry
Timothy Mason (1997)
- Record: found
- Abstract: found
- Article: not found
Discovery of dual function acridones as a new antimalarial chemotype.
Aaron Janowsky, Michael Winter, Sergio Wittlin … (2009)
- Record: found
- Abstract: found
- Article: not found
Interest of acridine derivatives in the anticancer chemotherapy.
M Demeunynck, A Martelli, F Charmantray (2001)