- Record: found
- Abstract: found
- Article: found
Carcinoembryonic antigen as a marker of radioresistance in colorectal cancer: a potential role of macrophages
Read this article at
Abstract
Background
We sought to identify the carcinoembryonic antigen (CEA) as a marker of radioresistance in rectal cancer.
Methods
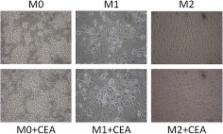
From July 1997 to January 2008, 104 patients with stage II or III rectal cancer who were treated with post-operative radiotherapy (PORT) were included in this study. The doses of radiotherapy ranged from 45 to 54.6 Gy. The CEA levels were measured before surgery. We analyzed the actuarial rates of overall survival (OS), distant metastasis (DM), and local recurrence (LR) using Kaplan-Meier curves. Multivariate analyses were performed with Cox regression models. We used THP-1 monocyte cell lines for macrophage differentiation (M0, M1 or M2). The RNA extracted from the macrophages was analyzed via a genomic method in the core laboratory. The radiosensitivities of CEA-rich LS1034 cells were compared between cells with and without the conditioned media from CEA-stimulated macrophages.
Results
Preoperative CEA levels ≥10 ng/mL were independent predictive factors for OS ( p = 0.005), DM ( p = 0.026), and LR ( p = 0.004). The OS rates among the patients with pretreatment CEA levels < 10 ng/mL and ≥10 ng/mL were 64.5% and 35.9% ( p = 0.004), respectively. The corresponding rates of DM were 40.6% and 73.1% ( p = 0.024). The corresponding rates of LR were 6.6% and 33.9% ( p = 0.002). In the M0 macrophages, exogenous CEA elicited a dose-response relationship with M2 differentiation. In the CEA-stimulated M0 cells, some mRNAs were upregulated by as much as 5-fold, including MMP12, GDF15, and JAG1. In the CEA-stimulated M2 cells, a 4-fold up-regulation of GADD45G mRNA was noted. The conditioned media from the CEA-stimulated M2 cells elicited an increase in the numbers of LS180, SW620, and LS1034 cells after irradiation. CEA caused the M2 differentiation of the macrophages.
Conclusion
Pretreatment CEA levels ≥10 ng/mL are a significant risk factor for OS, DM, and LR following PORT for rectal cancer. CEA causes radioresistance in the presence of M2 macrophages. More comprehensive examinations prior to surgery and intensive adjuvant therapy are suggested for patients with CEA levels ≥10 ng/mL. Further studies of these mechanisms are needed.
Related collections
Most cited references32

- Record: found
- Abstract: found
- Article: found
Macrophages in Tumor Microenvironments and the Progression of Tumors
- Record: found
- Abstract: found
- Article: not found
Endothelial cells promote the colorectal cancer stem cell phenotype through a soluble form of Jagged-1.
- Record: found
- Abstract: found
- Article: not found