- Record: found
- Abstract: found
- Article: found
Mechanisms of Vascular Calcification: The Pivotal Role of Pyruvate Dehydrogenase Kinase 4
Read this article at
Abstract
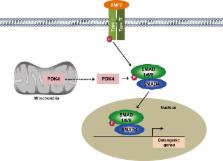
Vascular calcification, abnormal mineralization of the vessel wall, is frequently associated with aging, atherosclerosis, diabetes mellitus, and chronic kidney disease. Vascular calcification is a key risk factor for many adverse clinical outcomes, including ischemic cardiac events and subsequent cardiovascular mortality. Vascular calcification was long considered to be a passive degenerative process, but it is now recognized as an active and highly regulated process similar to bone formation. However, despite numerous studies on the pathogenesis of vascular calcification, the mechanisms driving this process remain poorly understood. Pyruvate dehydrogenase kinases (PDKs) play an important role in the regulation of cellular metabolism and mitochondrial function. Recent studies show that PDK4 is an attractive therapeutic target for the treatment of various metabolic diseases. In this review, we summarize our current knowledge regarding the mechanisms of vascular calcification and describe the role of PDK4 in the osteogenic differentiation of vascular smooth muscle cells and development of vascular calcification. Further studies aimed at understanding the molecular mechanisms of vascular calcification will be critical for the development of novel therapeutic strategies.
Related collections
Most cited references74
- Record: found
- Abstract: found
- Article: not found
Human vascular smooth muscle cells undergo vesicle-mediated calcification in response to changes in extracellular calcium and phosphate concentrations: a potential mechanism for accelerated vascular calcification in ESRD.
- Record: found
- Abstract: found
- Article: not found
BMP receptor signaling: transcriptional targets, regulation of signals, and signaling cross-talk.
Author and article information
Comments
Comment on this article
 Smart Citations
Smart CitationsSee how this article has been cited at scite.ai
scite shows how a scientific paper has been cited by providing the context of the citation, a classification describing whether it supports, mentions, or contrasts the cited claim, and a label indicating in which section the citation was made.