- Record: found
- Abstract: found
- Article: found
Vascular Calcification—New Insights into Its Mechanism
Read this article at
Abstract
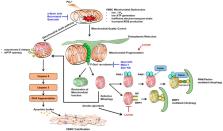
Vascular calcification (VC), which is categorized by intimal and medial calcification, depending on the site(s) involved within the vessel, is closely related to cardiovascular disease. Specifically, medial calcification is prevalent in certain medical situations, including chronic kidney disease and diabetes. The past few decades have seen extensive research into VC, revealing that the mechanism of VC is not merely a consequence of a high-phosphorous and -calcium milieu, but also occurs via delicate and well-organized biologic processes, including an imbalance between osteochondrogenic signaling and anticalcific events. In addition to traditionally established osteogenic signaling, dysfunctional calcium homeostasis is prerequisite in the development of VC. Moreover, loss of defensive mechanisms, by microorganelle dysfunction, including hyper-fragmented mitochondria, mitochondrial oxidative stress, defective autophagy or mitophagy, and endoplasmic reticulum (ER) stress, may all contribute to VC. To facilitate the understanding of vascular calcification, across any number of bioscientific disciplines, we provide this review of a detailed updated molecular mechanism of VC. This encompasses a vascular smooth muscle phenotypic of osteogenic differentiation, and multiple signaling pathways of VC induction, including the roles of inflammation and cellular microorganelle genesis.
Related collections
Most cited references246
- Record: found
- Abstract: not found
- Article: not found
Mitochondrial dynamics--mitochondrial fission and fusion in human diseases.
- Record: found
- Abstract: found
- Article: not found
Cytochrome C-mediated apoptosis.
- Record: found
- Abstract: found
- Article: not found